Etripamil Nasal Spray for Conversion of Repeated Spontaneous Episodes of Paroxysmal Supraventricular Tachycardia During Long-Term Follow-Up: Results From the NODE-302 Study
- PMID: 37753718
- PMCID: PMC10727262
- DOI: 10.1161/JAHA.122.028227
Etripamil Nasal Spray for Conversion of Repeated Spontaneous Episodes of Paroxysmal Supraventricular Tachycardia During Long-Term Follow-Up: Results From the NODE-302 Study
Abstract
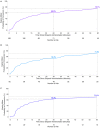
Background Self-administration of investigational intranasal L-type calcium channel blocker etripamil during paroxysmal supraventricular tachycardia (PSVT) appeared safe and well-tolerated in the phase 3 NODE-301 (Multi-Centre, Randomized, Double-Blind, Placebo-Controlled, Efficacy, and Safety Study of Etripamil Nasal Spray for the Termination of Spontaneous Episodes of Paroxysmal Supraventricular Tachycardia) trial of adults with sustained atrioventricular nodal-dependent PSVT. The NODE-302 open-label extension further characterized etripamil safety and efficacy. Methods and Results Eligible patients were monitored via self-applied cardiac monitoring system for 5 hours after etripamil self-administration. The primary end point was time-to-conversion of positively adjudicated PSVT to sinus rhythm after etripamil treatment. Probability of conversion to sinus rhythm was reported via Kaplan-Meier plot. Adverse events were based on self-reported symptoms and clinical evaluations. Among 169 patients enrolled, 105 self-administered etripamil ≥1 time for perceived PSVT (median [range], 232 [8-584] days' follow-up). Probability of conversion within 30 minutes of etripamil was 60.2% (median time to conversion, 15.5 minutes) among 188 PSVT episodes (92 patients) positively adjudicated as atrioventricular nodal dependent by independent ECG analysis. Among 40 patients who self-treated 2 episodes, 75% had a significantly consistent response by 30 minutes; 9 did not convert on either episode, and 21 converted on both episodes (χ2=8.09; P=0.0045). Forty-five of 105 patients (42.9%) had ≥1 treatment-emergent adverse event, generally transient and mild-to-moderate, including nasal congestion (14.3%), nasal discomfort (14.3%), or rhinorrhea (12.4%). No serious cardiac safety events were observed within 24 hours of etripamil. Conclusions In this extension study, investigational etripamil nasal spray was well tolerated for self-treating recurrent episodes of PSVT without medical supervision. Registration URL: https://www.clinicaltrials.gov; Unique identifier: NCT03635996.
Keywords: NODE‐302; etripamil; paroxysmal supraventricular tachycardia; self‐administered.
Figures

References
-
- Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomstrom‐Lundqvist C, Calkins H, Corrado D, Deftereos SG, Diller GP, et al. 2019 ESC guidelines for the management of patients with supraventricular tachycardia. The task force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J. 2020;41:655–720. doi: 10.1093/eurheartj/ehz467 - DOI - PubMed
-
- Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, Estes NA III, Field ME, Goldberger ZD, Hammill SC, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Heart Rhythm. 2016;13:e92–e135. doi: 10.1016/j.hrthm.2015.09.018 - DOI - PubMed
-
- Alboni P, Tomasi C, Menozzi C, Bottoni N, Paparella N, Fuca G, Brignole M, Cappato R. Efficacy and safety of out‐of‐hospital self‐administered single‐dose oral drug treatment in the management of infrequent, well‐tolerated paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 2001;37:548–553. doi: 10.1016/s0735-1097(00)01128-1 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

