Urinary comprehensive genomic profiling predicts urothelial carcinoma recurrence and identifies responders to intravesical therapy
- PMID: 37753732
- PMCID: PMC10850796
- DOI: 10.1002/1878-0261.13530
Urinary comprehensive genomic profiling predicts urothelial carcinoma recurrence and identifies responders to intravesical therapy
Abstract
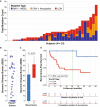
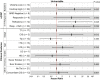
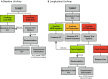
Intravesical therapy (IVT) is the standard of care to decrease risk of recurrence and progression for high-grade nonmuscle-invasive bladder cancer. However, post-IVT recurrence remains common and the ability to risk-stratify patients before or after IVT is limited. In this prospectively designed and accrued cohort study, we examine the utility of urinary comprehensive genomic profiling (uCGP) for predicting recurrence risk following transurethral resection of bladder tumor (TURBT) and evaluating longitudinal IVT response. Urine was collected before and after IVT instillation and uCGP testing was done using the UroAmp™ platform. Baseline uCGP following TURBT identified patients with high (61%) and low (39%) recurrence risk. At 24 months, recurrence-free survival (RFS) was 100% for low-risk and 45% for high-risk patients with a hazard ratio (HR) of 9.3. Longitudinal uCGP classified patients as minimal residual disease (MRD) Negative, IVT Responder, or IVT Refractory with 24-month RFS of 100%, 50%, and 32%, respectively. Compared with MRD Negative patients, IVT Refractory patients had a HR of 10.5. Collectively, uCGP enables noninvasive risk assessment of patients following TURBT and induction IVT. uCGP could inform surveillance cystoscopy schedules and identify high-risk patients in need of additional therapy.
Keywords: Bacillus Calmette-Guérin; bladder cancer; genomics; intravesical instillation; personalized medicine; risk assessment.
© 2023 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
VMC, DSF, PSL, CTW, BCM, KGP, VTB, and TGL report being employees and shareholders of Convergent Genomics. AJW discloses membership on the advisory boards of Urobiome Therapeutics and Pathnostics and funding from Pathnostics, VB Tech, the Craig H. Neilsen Foundation, and NIH. All other authors have no disclosures.
Figures

References
-
- Tobert CM, Nepple KG, McDowell BD, Charlton ME, Mott SL, Gruca TS, et al. Compliance with American Urological Association Guidelines for nonmuscle invasive bladder cancer remains poor: assessing factors associated with noncompliance and survival in a rural state. Urology. 2019;132:150–155. 10.1016/J.UROLOGY.2019.06.021 - DOI - PMC - PubMed
-
- Kamat AM, Georgieva MV, Song J, Bocharova I, Qian K, Guo A, et al. Disease progression among patients who receive available bladder preservation therapies after failure of BCG therapy in the SEER‐Medicare data. J Clin Oncol. 2020;38:453. 10.1200/JCO.2020.38.6_SUPPL.453 - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

