Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma
- PMID: 37753960
- PMCID: PMC10730063
- DOI: 10.1200/JCO.23.01696
Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma
Abstract
Purpose: The GMMG-CONCEPT trial investigated isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd) in transplant-eligible (TE) and transplant-noneligible (TNE) patients with newly diagnosed multiple myeloma (NDMM) with exclusively high-risk disease for whom prospective trials are limited, aiming to induce minimal residual disease (MRD) negativity.
Methods: This academic, investigator-initiated, multicenter, phase II trial enrolled patients with high-risk NDMM (HRNDMM) defined by mandatory International Staging System stage II/III combined with del17p, t(4;14), t(14;16), or more than three 1q21 copies as high-risk cytogenetic aberrations (HRCAs). Patients received Isa-KRd induction/consolidation and Isa-KR maintenance. TE patients received high-dose melphalan. TNE patients received two additional Isa-KRd cycles postinduction. This prespecified interim analysis (IA) reports the primary end point, MRD negativity (<10-5, next-generation flow), at the end of consolidation. The secondary end point was progression-free survival (PFS).
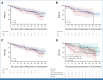
Results: Among 125 patients with HRNDMM (TE-intention-to-treat [ITT]-IA, 99; TNE-ITT, 26) of the IA population for the primary end point, the median age was 58 (TE-ITT-IA) and 74 (TNE-ITT) years. Del17p was the most common HRCA (TE, 44.4%; TNE, 42.3%); about one third of evaluable TE/TNE patients presented two or more HRCAs, respectively. The trial met its primary end point with MRD negativity rates after consolidation of 67.7% (TE) and 54.2% (TNE) of patients. Eighty-one of 99 TE-ITT-IA patients reached MRD negativity at any time point (81.8%). MRD negativity was sustained for ≥1 year in 62.6% of patients. With a median follow-up of 44 (TE) and 33 (TNE) months, median PFS was not reached in either arm.
Conclusion: Isa-KRd effectively induces high rates of sustainable MRD negativity in the difficult-to-treat HRNDMM population, regardless of transplant status, translating into a median PFS that was not yet reached after 44/33 months.
Trial registration: ClinicalTrials.gov NCT03104842.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures






References
-
- Mateos MV, Dimopoulos MA, Cavo M, et al. : Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 378:518-528, 2018 - PubMed
-
- Moreau P, Attal M, Hulin C, et al. : Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 394:29-38, 2019 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

