Whole-Body Photobiomodulation Therapy for Fibromyalgia: A Feasibility Trial
- PMID: 37753995
- PMCID: PMC10525895
- DOI: 10.3390/bs13090717
Whole-Body Photobiomodulation Therapy for Fibromyalgia: A Feasibility Trial
Abstract
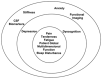
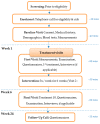
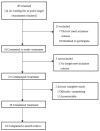
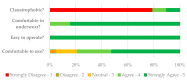
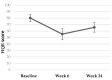
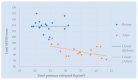
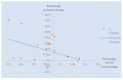
Effective treatment for fibromyalgia (FM) is lacking and further treatment options are needed. Photobiomodulation therapy (PBMT) represents one potential treatment option. Whilst favourable findings have been reported using localised PBMT, no investigations have established the value of whole-body PBMT for the complete set of symptom domains in FM. A single-arm feasibility study was conducted in accordance with CONSORT (Consolidated Standards of Reporting Trials) guidelines. A non-probability sampling method was used to access individuals with FM. The primary outcome measure was identified as the Revised Fibromyalgia Impact Questionnaire (FIQR). Forty-nine participants were screened and twenty-one trial participants entered the trial. Nineteen participants completed the intervention (18 whole-body PBMT sessions over approximately six weeks). Descriptive statistics and qualitative analysis was undertaken to represent feasibility outcomes. Acceptability of the trial device and processes were established. Outcome measures towards efficacy data were guided by core and peripheral OMERACT (outcomes measures in rheumatological clinical trials) domains, utilising a combination of participant-reported and performance-based outcome measures. Data for the embedded qualitative component of the trial were captured by participant-reported experience measures and audio-recorded semi-structured interviews. Positive changes were observed for FM-specific quality of life, pain, tenderness, stiffness, fatigue, sleep disturbance, anxiety, depression and cognitive impairment. Patient global assessment revealed improvements at 6 weeks, with continued effect at 24 weeks. FM-specific quality of life at 24 weeks remained improved compared with baseline scores. The findings provided evidence to support a full-scale trial and showed promise regarding potential efficacy of this novel non-invasive treatment in an FM population.
Keywords: chronic primary widespread pain; feasibility trial; fibromyalgia; photobiomodulation therapy; whole-body.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Whole-Body Photobiomodulation Therapy Propels the Fibromyalgia Patient into the Recomposition Phase: A Reflexive Thematic Analysis.Biomedicines. 2024 May 17;12(5):1116. doi: 10.3390/biomedicines12051116. Biomedicines. 2024. PMID: 38791077 Free PMC article.
-
Whole-body photobiomodulation therapy for chronic pain: a protocol for a feasibility trial.BMJ Open. 2022 Jun 29;12(6):e060058. doi: 10.1136/bmjopen-2021-060058. BMJ Open. 2022. PMID: 35768101 Free PMC article.
-
Outcomes of whole-body photobiomodulation on pain, quality of life, leisure physical activity, pain catastrophizing, kinesiophobia, and self-efficacy: a prospective randomized triple-blinded clinical trial with 6 months of follow-up.Front Neurosci. 2024 Jan 31;18:1264821. doi: 10.3389/fnins.2024.1264821. eCollection 2024. Front Neurosci. 2024. PMID: 38356644 Free PMC article.
-
Fibromyalgia syndrome module at OMERACT 9: domain construct.J Rheumatol. 2009 Oct;36(10):2318-29. doi: 10.3899/jrheum.090367. J Rheumatol. 2009. PMID: 19820221 Free PMC article.
-
Fibromyalgia syndrome.J Rheumatol. 2007 Jun;34(6):1415-25. J Rheumatol. 2007. PMID: 17552068
Cited by
-
Effects of photobiomodulation on multiple health outcomes: an umbrella review of randomized clinical trials.Syst Rev. 2025 Aug 6;14(1):160. doi: 10.1186/s13643-025-02902-3. Syst Rev. 2025. PMID: 40770824 Free PMC article.
-
Whole-Body Photobiomodulation Therapy Propels the Fibromyalgia Patient into the Recomposition Phase: A Reflexive Thematic Analysis.Biomedicines. 2024 May 17;12(5):1116. doi: 10.3390/biomedicines12051116. Biomedicines. 2024. PMID: 38791077 Free PMC article.
-
The Fibromyalgia Decomposition Phenomenon: A Reflexive Thematic Analysis.Behav Sci (Basel). 2024 Jan 11;14(1):47. doi: 10.3390/bs14010047. Behav Sci (Basel). 2024. PMID: 38247699 Free PMC article.
References
LinkOut - more resources
Full Text Sources

