Post-Assay Chemical Enhancement for Highly Sensitive Lateral Flow Immunoassays: A Critical Review
- PMID: 37754100
- PMCID: PMC10526817
- DOI: 10.3390/bios13090866
Post-Assay Chemical Enhancement for Highly Sensitive Lateral Flow Immunoassays: A Critical Review
Abstract
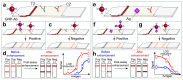
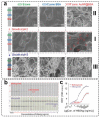
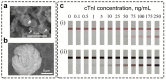
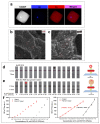
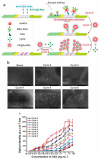
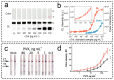
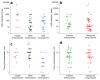
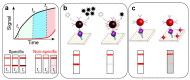
Lateral flow immunoassay (LFIA) has found a broad application for testing in point-of-care (POC) settings. LFIA is performed using test strips-fully integrated multimembrane assemblies containing all reagents for assay performance. Migration of liquid sample along the test strip initiates the formation of labeled immunocomplexes, which are detected visually or instrumentally. The tradeoff of LFIA's rapidity and user-friendliness is its relatively low sensitivity (high limit of detection), which restricts its applicability for detecting low-abundant targets. An increase in LFIA's sensitivity has attracted many efforts and is often considered one of the primary directions in developing immunochemical POC assays. Post-assay enhancements based on chemical reactions facilitate high sensitivity. In this critical review, we explain the performance of post-assay chemical enhancements, discuss their advantages, limitations, compared limit of detection (LOD) improvements, and required time for the enhancement procedures. We raise concerns about the performance of enhanced LFIA and discuss the bottlenecks in the existing experiments. Finally, we suggest the experimental workflow for step-by-step development and validation of enhanced LFIA. This review summarizes the state-of-art of LFIA with chemical enhancement, offers ways to overcome existing limitations, and discusses future outlooks for highly sensitive testing in POC conditions.
Keywords: antibodies; highly sensitive detection; immunochromatography; nanoparticles; nanozymes; point-of-care testing; signal amplification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

