Electrochemical Biosensors for the Detection of Antibiotics in Milk: Recent Trends and Future Perspectives
- PMID: 37754101
- PMCID: PMC10527191
- DOI: 10.3390/bios13090867
Electrochemical Biosensors for the Detection of Antibiotics in Milk: Recent Trends and Future Perspectives
Abstract
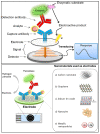
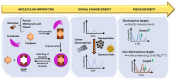
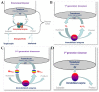
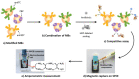
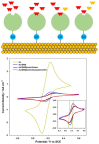
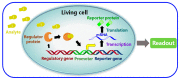
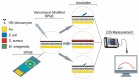
Antibiotics have emerged as ground-breaking medications for the treatment of infectious diseases, but due to the excessive use of antibiotics, some drugs have developed resistance to microorganisms. Because of their structural complexity, most antibiotics are excreted unchanged, polluting the water, soil, and natural resources. Additionally, food items are being polluted through the widespread use of antibiotics in animal feed. The normal concentrations of antibiotics in environmental samples typically vary from ng to g/L. Antibiotic residues in excess of these values can pose major risks the development of illnesses and infections/diseases. According to estimates, 300 million people will die prematurely in the next three decades (by 2050), and the WHO has proclaimed "antibiotic resistance" to be a severe economic and sociological hazard to public health. Several antibiotics have been recognised as possible environmental pollutants (EMA) and their detection in various matrices such as food, milk, and environmental samples is being investigated. Currently, chromatographic techniques coupled with different detectors (e.g., HPLC, LC-MS) are typically used for antibiotic analysis. Other screening methods include optical methods, ELISA, electrophoresis, biosensors, etc. To minimise the problems associated with antibiotics (i.e., the development of AMR) and the currently available analytical methods, electrochemical platforms have been investigated, and can provide a cost-effective, rapid and portable alternative. Despite the significant progress in this field, further developments are necessary to advance electrochemical sensors, e.g., through the use of multi-functional nanomaterials and advanced (bio)materials to ensure efficient detection, sensitivity, portability, and reliability. This review summarises the use of electrochemical biosensors for the detection of antibiotics in milk/milk products and presents a brief introduction to antibiotics and AMR followed by developments in the field of electrochemical biosensors based on (i) immunosensor, (ii) aptamer (iii) MIP, (iv) enzyme, (v) whole-cell and (vi) direct electrochemical approaches. The role of nanomaterials and sensor fabrication is discussed wherever necessary. Finally, the review discusses the challenges encountered and future perspectives. This review can serve as an insightful source of information, enhancing the awareness of the role of electrochemical biosensors in providing information for the preservation of the health of the public, of animals, and of our environment, globally.
Keywords: AMR; MIPs; antibiotics; aptamers and enzymes; biosensors; electrochemical instrumentation; immunosensors; milk; nanomaterials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













References
-
- Sharma A., Istamboulie G., Hayat A., Catanante G., Bhand S., Marty J.L. Disposable and portable aptamer functionalized impedimetric sensor for detection of kanamycin residue in milk sample. Sens. Actuators B Chem. 2017;245:507–515.
-
- Ben Y., Fu C., Hu M., Liu L., Wong M.H., Zheng C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019;169:483–493. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

