Microlens-Assisted Light-Scattering Imaging of Plasmonic Nanoparticles at the Single Particle Level
- PMID: 37754105
- PMCID: PMC10526809
- DOI: 10.3390/bios13090871
Microlens-Assisted Light-Scattering Imaging of Plasmonic Nanoparticles at the Single Particle Level
Abstract
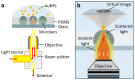
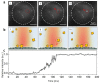
We present a microlens-assisted imaging approach to record the scattering light of plasmonic nanoparticles at the single particle level. The microlens can significantly enhance the backscattering of visible light from individual plasmonic nanoparticles by several dozen folds, and single gold nanoparticles with a diameter as low as 60 nm can be imaged under a conventional optical microscope. This can benefit from a significant increase in the scattering intensity afforded by the microlens, meaning that the imaging of gold nanoparticles at a high temporal resolution (up to 5000 Hz) can be achieved, which is fast enough to record single particle adhesion events on the substrate. This research presents a fast and efficient means of acquiring scattering light from plasmonic nanoparticles, which has great potential to develop plasmonic nanoparticle-based biosensors and investigate a wide range of plasmonic nanoparticle-based fast interaction processes.
Keywords: dielectric microsphere; high temporal resolution; light-scattering imaging; microlens; plasmonic nanoparticle; single-nanoparticle imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Application of Gold Nanoparticle to Plasmonic Biosensors.Int J Mol Sci. 2018 Jul 11;19(7):2021. doi: 10.3390/ijms19072021. Int J Mol Sci. 2018. PMID: 29997363 Free PMC article. Review.
-
Individual Plasmonic Nanoprobes for Biosensing and Bioimaging: Recent Advances and Perspectives.Small. 2021 Feb;17(8):e2004287. doi: 10.1002/smll.202004287. Epub 2021 Feb 1. Small. 2021. PMID: 33522074 Review.
-
Single plasmonic nanoparticles for ultrasensitive DNA sensing: From invisible to visible.Biosens Bioelectron. 2016 May 15;79:266-72. doi: 10.1016/j.bios.2015.12.027. Epub 2015 Dec 15. Biosens Bioelectron. 2016. PMID: 26720918
-
A rapid readout for many single plasmonic nanoparticles using dark-field microscopy and digital color analysis.Biosens Bioelectron. 2018 Oct 15;117:530-536. doi: 10.1016/j.bios.2018.06.066. Epub 2018 Jul 5. Biosens Bioelectron. 2018. PMID: 29982124
-
Measurement of Scattering Nonlinearities from a Single Plasmonic Nanoparticle.J Vis Exp. 2016 Jan 3;(107):53338. doi: 10.3791/53338. J Vis Exp. 2016. PMID: 26780248 Free PMC article.
Cited by
-
Metasurface-Coated Liquid Microlens for Super Resolution Imaging.Micromachines (Basel). 2024 Dec 27;16(1):25. doi: 10.3390/mi16010025. Micromachines (Basel). 2024. PMID: 39858681 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

