SERS-Based Optical Nanobiosensors for the Detection of Alzheimer's Disease
- PMID: 37754114
- PMCID: PMC10526933
- DOI: 10.3390/bios13090880
SERS-Based Optical Nanobiosensors for the Detection of Alzheimer's Disease
Abstract
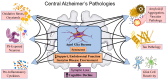
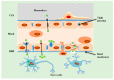
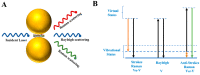
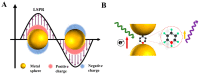
Alzheimer's disease (AD) is a leading cause of dementia, impacting millions worldwide. However, its complex neuropathologic features and heterogeneous pathophysiology present significant challenges for diagnosis and treatment. To address the urgent need for early AD diagnosis, this review focuses on surface-enhanced Raman scattering (SERS)-based biosensors, leveraging the excellent optical properties of nanomaterials to enhance detection performance. These highly sensitive and noninvasive biosensors offer opportunities for biomarker-driven clinical diagnostics and precision medicine. The review highlights various types of SERS-based biosensors targeting AD biomarkers, discussing their potential applications and contributions to AD diagnosis. Specific details about nanomaterials and targeted AD biomarkers are provided. Furthermore, the future research directions and challenges for improving AD marker detection using SERS sensors are outlined.
Keywords: Alzheimer’s disease; SERS; biosensor; disease diagnosis; fluid biomarkers; nanoparticles.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures




Similar articles
-
Highly sensitive surface-enhanced Raman scattering-based immunosensor incorporating half antibody-fragment for quantitative detection of Alzheimer's disease biomarker in blood.Anal Chim Acta. 2022 Feb 22;1195:339445. doi: 10.1016/j.aca.2022.339445. Epub 2022 Jan 5. Anal Chim Acta. 2022. PMID: 35090659
-
Early-stage Alzheimer's disease profiling in blood achieved by multiplexing aptamer-SERS biosensors.Biosens Bioelectron. 2025 Jan 15;268:116907. doi: 10.1016/j.bios.2024.116907. Epub 2024 Nov 2. Biosens Bioelectron. 2025. PMID: 39509994
-
Plasmonic Nanoparticles as Optical Sensing Probes for the Detection of Alzheimer's Disease.Sensors (Basel). 2021 Mar 16;21(6):2067. doi: 10.3390/s21062067. Sensors (Basel). 2021. PMID: 33809416 Free PMC article. Review.
-
Nanomaterial-based Optical and Electrochemical Biosensors for Amyloid beta and Tau: Potential for early diagnosis of Alzheimer's Disease.Expert Rev Mol Diagn. 2021 Feb;21(2):175-193. doi: 10.1080/14737159.2021.1887732. Epub 2021 Mar 1. Expert Rev Mol Diagn. 2021. PMID: 33560154
-
A Concise Overview of Biosensing Technologies for the Detection of Alzheimer's Disease Biomarkers.Curr Pharm Biotechnol. 2022;23(5):634-644. doi: 10.2174/2666796702666210709122407. Curr Pharm Biotechnol. 2022. PMID: 34250871 Review.
Cited by
-
Advancing Brain Research through Surface-Enhanced Raman Spectroscopy (SERS): Current Applications and Future Prospects.Biosensors (Basel). 2024 Jan 10;14(1):33. doi: 10.3390/bios14010033. Biosensors (Basel). 2024. PMID: 38248410 Free PMC article. Review.
-
Optical biosensors for diagnosing neurodegenerative diseases.NPJ Biosens. 2025;2(1):20. doi: 10.1038/s44328-025-00040-3. Epub 2025 May 2. NPJ Biosens. 2025. PMID: 40322247 Free PMC article. Review.
-
Nanobiosensors in neurodegenerative disease diagnosis: A promising pathway for early detection.Digit Health. 2025 May 14;11:20552076251342457. doi: 10.1177/20552076251342457. eCollection 2025 Jan-Dec. Digit Health. 2025. PMID: 40376568 Free PMC article. Review.
-
Optical Bioassays Based on the Signal Amplification of Redox Cycling.Biosensors (Basel). 2024 May 24;14(6):269. doi: 10.3390/bios14060269. Biosensors (Basel). 2024. PMID: 38920573 Free PMC article. Review.
-
Bridging pandemic and oncology challenges: Surface-enhanced Raman spectroscopy in the fight against COVID-19 and cancer.Sci Prog. 2025 Apr-Jun;108(2):368504251342977. doi: 10.1177/00368504251342977. Epub 2025 May 30. Sci Prog. 2025. PMID: 40443291 Free PMC article. Review.
References
-
- Ferretti M.T., Iulita M.F., Cavedo E., Chiesa P.A., Schumacher Dimech A., Santuccione Chadha A., Baracchi F., Girouard H., Misoch S., Giacobini E., et al. Sex differences in Alzheimer disease-the gateway to precision medicine. Nat. Rev. Neurol. 2018;14:457–469. doi: 10.1038/s41582-018-0032-9. - DOI - PubMed
-
- Jiang F., Mishra S.R., Shrestha N., Ozaki A., Virani S.S., Bright T., Kuper H., Zhou C., Zhu D. Association between hearing aid use and all-cause and cause-specific dementia: An analysis of the UK Biobank cohort. Lancet Public Health. 2023;8:e329–e338. doi: 10.1016/S2468-2667(23)00048-8. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

