Recent Uses of Paper Microfluidics in Isothermal Nucleic Acid Amplification Tests
- PMID: 37754119
- PMCID: PMC10526735
- DOI: 10.3390/bios13090885
Recent Uses of Paper Microfluidics in Isothermal Nucleic Acid Amplification Tests
Abstract
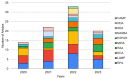
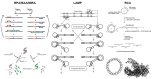
Isothermal nucleic acid amplification tests have recently gained popularity over polymerase chain reaction (PCR), as they only require a constant temperature and significantly simplify nucleic acid amplification. Recently, numerous attempts have been made to incorporate paper microfluidics into these isothermal amplification tests. Paper microfluidics (including lateral flow strips) have been used to extract nucleic acids, amplify the target gene, and detect amplified products, all toward automating the process. We investigated the literature from 2020 to the present, i.e., since the onset of the COVID-19 pandemic, during which a significant surge in isothermal amplification tests has been observed. Paper microfluidic detection has been used extensively for recombinase polymerase amplification (RPA) and its related methods, along with loop-mediated isothermal amplification (LAMP) and rolling circle amplification (RCA). Detection was conducted primarily with colorimetric and fluorometric methods, although a few publications demonstrated flow distance- and surface-enhanced Raman spectroscopic (SERS)-based detection. A good number of publications could be found that demonstrated both amplification and detection on paper microfluidic platforms. A small number of publications could be found that showed extraction or all three procedures (i.e., fully integrated systems) on paper microfluidic platforms, necessitating the need for future work.
Keywords: lateral flow immunochromatographic assay; loop-mediated isothermal amplification; microfluidic paper-based analytic device; recombinase polymerase amplification; rolling circle amplification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Digital Recombinase Polymerase Amplification, Digital Loop-Mediated Isothermal Amplification, and Digital CRISPR-Cas Assisted Assay: Current Status, Challenges, and Perspectives.Small. 2023 Dec;19(49):e2303398. doi: 10.1002/smll.202303398. Epub 2023 Aug 23. Small. 2023. PMID: 37612816 Review.
-
Isothermal amplification-based microfluidic devices for detecting foodborne pathogens: a review.Anal Methods. 2024 Feb 22;16(8):1150-1157. doi: 10.1039/d3ay02039h. Anal Methods. 2024. PMID: 38323529 Review.
-
Recombinase polymerase amplification integrated with microfluidics for nucleic acid testing at point of care.Talanta. 2022 Apr 1;240:123209. doi: 10.1016/j.talanta.2022.123209. Epub 2022 Jan 4. Talanta. 2022. PMID: 35026642 Review.
-
Prospects of Microfluidic Technology in Nucleic Acid Detection Approaches.Biosensors (Basel). 2023 May 27;13(6):584. doi: 10.3390/bios13060584. Biosensors (Basel). 2023. PMID: 37366949 Free PMC article. Review.
-
A microfluidic-integrated lateral flow recombinase polymerase amplification (MI-IF-RPA) assay for rapid COVID-19 detection.Lab Chip. 2021 May 18;21(10):2019-2026. doi: 10.1039/d0lc01222j. Lab Chip. 2021. PMID: 34008614
Cited by
-
Chemical Trends in Sample Preparation for Nucleic Acid Amplification Testing (NAAT): A Review.Biosensors (Basel). 2023 Nov 10;13(11):980. doi: 10.3390/bios13110980. Biosensors (Basel). 2023. PMID: 37998155 Free PMC article. Review.
-
Capillary flow velocity-based length identification of PCR and RPA products on paper microfluidic chips.Biosens Bioelectron. 2025 Jan 1;267:116861. doi: 10.1016/j.bios.2024.116861. Epub 2024 Oct 25. Biosens Bioelectron. 2025. PMID: 39455308
-
Development of a Paper-Based Microfluidic Chip for Point-of-Care Detection of PEDV.Vet Sci. 2025 Apr 30;12(5):427. doi: 10.3390/vetsci12050427. Vet Sci. 2025. PMID: 40431520 Free PMC article.
-
Microfluidic Paper-Based Devices.Micromachines (Basel). 2025 Mar 6;16(3):307. doi: 10.3390/mi16030307. Micromachines (Basel). 2025. PMID: 40141918 Free PMC article.
-
Facile Splint-Free Circularization of ssDNA with T4 DNA Ligase by Redesigning the Linear Substrate to Form an Intramolecular Dynamic Nick.Biomolecules. 2024 Aug 18;14(8):1027. doi: 10.3390/biom14081027. Biomolecules. 2024. PMID: 39199414 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

