Development of a Multiplex HIV/TB Diagnostic Assay Based on the Microarray Technology
- PMID: 37754128
- PMCID: PMC10526232
- DOI: 10.3390/bios13090894
Development of a Multiplex HIV/TB Diagnostic Assay Based on the Microarray Technology
Abstract
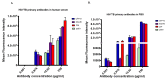
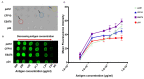
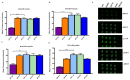
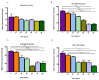
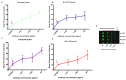
Currently there are diagnostic tests available for human immunodeficiency virus (HIV) and tuberculosis (TB); however, they are still diagnosed separately, which can delay treatment in cases of co-infection. Here we report on a multiplex microarray technology for the detection of HIV and TB antibodies using p24 as well as TB CFP10, ESAT6 and pstS1 antigens on epoxy-silane slides. To test this technology for antigen-antibody interactions, immobilized antigens were exposed to human sera spiked with physiological concentrations of primary antibodies, followed by secondary antibodies conjugated to a fluorescent reporter. HIV and TB antibodies were captured with no cross-reactivity observed. The sensitivity of the slides was compared to that of high-binding plates. We found that the slides were more sensitive, with the detection limit being 0.000954 µg/mL compared to 4.637 µg/mL for the plates. Furthermore, stability studies revealed that the immobilized antigens could be stored dry for at least 90 days and remained stable across all pH and temperatures assessed, with pH 7.4 and 25 °C being optimal. The data collectively suggested that the HIV/TB multiplex detection technology we developed has the potential for use to diagnose HIV and TB co-infection, and thus can be developed further for the purpose.
Keywords: HIV-1 p24; M.tb CFP10; M.tb ESAT6; M.tb pstS1; antibody; antigen; diagnosis; multiplex microarray.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






Similar articles
-
IgG subclasses' response to a set of mycobacterial antigens in different stages of Mycobacterium tuberculosis infection.Tuberculosis (Edinb). 2018 Jan;108:70-76. doi: 10.1016/j.tube.2017.10.010. Epub 2017 Oct 31. Tuberculosis (Edinb). 2018. PMID: 29523330
-
Close contact interferon-gamma response to the new PstS1(285-374):CPF10: a preliminary 1-year follow-up study.BMC Res Notes. 2017 Jan 23;10(1):59. doi: 10.1186/s13104-016-2360-4. BMC Res Notes. 2017. PMID: 28114976 Free PMC article.
-
Sensitive Blood-Based Detection of HIV-1 and Mycobacterium tuberculosis Peptides for Disease Diagnosis by Immuno-Affinity Liquid Chromatography-Tandem Mass Spectrometry: A Method Development and Proof-of-Concept Study.Clin Chem. 2023 Dec 1;69(12):1409-1419. doi: 10.1093/clinchem/hvad173. Clin Chem. 2023. PMID: 37956323 Free PMC article.
-
Tuberculosis and HIV coinfection.Semin Respir Crit Care Med. 2013 Feb;34(1):32-43. doi: 10.1055/s-0032-1333469. Epub 2013 Mar 4. Semin Respir Crit Care Med. 2013. PMID: 23460004 Review.
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
References
-
- UNIAIDS Global HIV & AIDS Statistics—Fact Sheet. 2021, pp. 1–7. [(accessed on 20 September 2022)]. Available online: https://www.unaids.org/en/resources/fact-sheet.
-
- Antony B., Tiewsoh J.B.A., Boloor R. HIV-TB co-infection with clinical presentation, diagnosis, treatment, outcome and its relation to CD4 count, a cross-sectional study in a tertiary care hospital in coastal Karnataka. J. Fam. Med. Prim. Care. 2020;9:1160–1165. doi: 10.4103/jfmpc.jfmpc_950_19. - DOI - PMC - PubMed
-
- Dewan R., Anuradha S., Khanna A., Garg S., Singla S., Ish P., Agarwal S., Narayana H.A., Hanif M., Singh H., et al. Role of cartridge-based nucleic acid amplification test (CBNAAT) for early diagnosis of pulmonary tuberculosis in HIV. Indian Acad. Clin. Med. 2015;16:114–117.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

