3D-Printed Tumor-on-a-Chip Model for Investigating the Effect of Matrix Stiffness on Glioblastoma Tumor Invasion
- PMID: 37754172
- PMCID: PMC10526170
- DOI: 10.3390/biomimetics8050421
3D-Printed Tumor-on-a-Chip Model for Investigating the Effect of Matrix Stiffness on Glioblastoma Tumor Invasion
Abstract
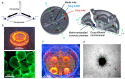
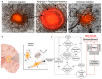
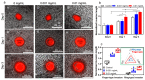
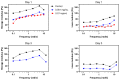
Glioblastoma multiform (GBM) tumor progression has been recognized to be correlated with extracellular matrix (ECM) stiffness. Dynamic variation of tumor ECM is primarily regulated by a family of enzymes which induce remodeling and degradation. In this paper, we investigated the effect of matrix stiffness on the invasion pattern of human glioblastoma tumoroids. A 3D-printed tumor-on-a-chip platform was utilized to culture human glioblastoma tumoroids with the capability of evaluating the effect of stiffness on tumor progression. To induce variations in the stiffness of the collagen matrix, different concentrations of collagenase were added, thereby creating an inhomogeneous collagen concentration. To better understand the mechanisms involved in GBM invasion, an in silico hybrid mathematical model was used to predict the evolution of a tumor in an inhomogeneous environment, providing the ability to study multiple dynamic interacting variables. The model consists of a continuum reaction-diffusion model for the growth of tumoroids and a discrete model to capture the migration of single cells into the surrounding tissue. Results revealed that tumoroids exhibit two distinct patterns of invasion in response to the concentration of collagenase, namely ring-type and finger-type patterns. Moreover, higher concentrations of collagenase resulted in greater invasion lengths, confirming the strong dependency of tumor behavior on the stiffness of the surrounding matrix. The agreement between the experimental results and the model's predictions demonstrates the advantages of this approach in investigating the impact of various extracellular matrix characteristics on tumor growth and invasion.
Keywords: 3D-printing; glioblastoma; in silico model; tumor-on-a-chip.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using PEG-based hydrogels.Mol Pharm. 2014 Jul 7;11(7):2115-25. doi: 10.1021/mp5000828. Epub 2014 Apr 29. Mol Pharm. 2014. PMID: 24712441
-
The mode and dynamics of glioblastoma cell invasion into a decellularized tissue-derived extracellular matrix-based three-dimensional tumor model.Sci Rep. 2018 Mar 15;8(1):4608. doi: 10.1038/s41598-018-22681-3. Sci Rep. 2018. PMID: 29545552 Free PMC article.
-
Decellularized brain extracellular matrix slice glioblastoma culture model recapitulates the interaction between cells and the extracellular matrix without a nutrient-oxygen gradient interference.Acta Biomater. 2023 Mar 1;158:132-150. doi: 10.1016/j.actbio.2022.12.044. Epub 2022 Dec 22. Acta Biomater. 2023. PMID: 36565784
-
3D Cancer Models: The Need for a Complex Stroma, Compartmentalization and Stiffness.Front Bioeng Biotechnol. 2021 Apr 12;9:660502. doi: 10.3389/fbioe.2021.660502. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33912551 Free PMC article. Review.
-
Mechanisms of Invasion in Glioblastoma: Extracellular Matrix, Ca2+ Signaling, and Glutamate.Front Cell Neurosci. 2021 Jun 2;15:663092. doi: 10.3389/fncel.2021.663092. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34149360 Free PMC article. Review.
Cited by
-
Applications of 3D Bioprinting Technology to Brain Cells and Brain Tumor Models: Special Emphasis to Glioblastoma.ACS Biomater Sci Eng. 2024 May 13;10(5):2616-2635. doi: 10.1021/acsbiomaterials.3c01569. Epub 2024 Apr 25. ACS Biomater Sci Eng. 2024. PMID: 38664996 Free PMC article. Review.
-
3D Bioprinting in Cancer Modeling and Biomedicine: From Print Categories to Biological Applications.ACS Omega. 2024 Oct 25;9(44):44076-44100. doi: 10.1021/acsomega.4c06051. eCollection 2024 Nov 5. ACS Omega. 2024. PMID: 39524656 Free PMC article. Review.
-
Hybrid-integrated devices for mimicking malignant brain tumors ("tumor-on-a-chip") for in vitro development of targeted drug delivery and personalized therapy approaches.Front Med (Lausanne). 2024 Nov 19;11:1452298. doi: 10.3389/fmed.2024.1452298. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39629230 Free PMC article. Review.
-
Biosensor-Enhanced Organ-on-a-Chip Models for Investigating Glioblastoma Tumor Microenvironment Dynamics.Sensors (Basel). 2024 Apr 30;24(9):2865. doi: 10.3390/s24092865. Sensors (Basel). 2024. PMID: 38732975 Free PMC article. Review.
-
Tumoroid Model Reveals Synergistic Impairment of Metabolism by Iron Chelators and Temozolomide in Chemo-Resistant Patient-derived Glioblastoma Cells.Adv Sci (Weinh). 2025 May;12(20):e2412505. doi: 10.1002/advs.202412505. Epub 2025 Apr 26. Adv Sci (Weinh). 2025. PMID: 40285641 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

