In Silico Investigation of AKT2 Gene and Protein Abnormalities Reveals Potential Association with Insulin Resistance and Type 2 Diabetes
- PMID: 37754255
- PMCID: PMC10528407
- DOI: 10.3390/cimb45090471
In Silico Investigation of AKT2 Gene and Protein Abnormalities Reveals Potential Association with Insulin Resistance and Type 2 Diabetes
Abstract
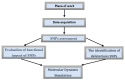
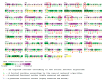
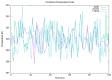
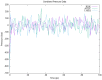
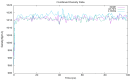
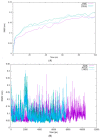
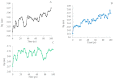
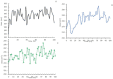
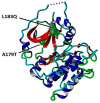
Type 2 diabetes (T2D) develops from insulin resistance (IR) and the dysfunction of pancreatic beta cells. The AKT2 protein is very important for the protein signaling pathway, and the non-synonymous SNP (nsSNPs) in AKT2 gene may be associated with T2D. nsSNPs can result in alterations in protein stability, enzymatic activity, or binding specificity. The objective of this study was to investigate the effect of nsSNPs on the AKT2 protein structure and function that may result in the induction of IR and T2D. The study identified 20 variants that were considered to be the most deleterious based on a range of analytical tools included (SIFT, PolyPhen2, Mut-pred, SNAP2, PANTHER, PhD-SNP, SNP&Go, MUpro, Cosurf, and I-Mut). Two mutations, p.A179T and p.L183Q, were selected for further investigation based on their location within the protein as determined by PyMol. The results indicated that mutations, p.A179T and p.L183Q alter the protein stability and functional characteristics, which could potentially affect its function. In order to conduct a more in-depth analysis of these effects, a molecular dynamics simulation was performed for wildtype AKT2 and the two mutants (p.A179T and p.L183Q). The simulation evaluated various parameters, including temperature, pressure, density, RMSD, RMSF, SASA, and Region, over a period of 100 ps. According to the simulation results, the wildtype AKT2 protein demonstrated higher stability in comparison to the mutant variants. The mutations p.A179T and p.L183Q were found to cause a reduction in both protein stability and functionality. These findings underscore the significance of the effects of nsSNPs (mutations p.A179T and p.L183Q) on the structure and function of AKT2 that may lead to IR and T2D. Nevertheless, they require further verifications in future protein functional, protein-protein interaction, and large-scale case-control studies. When verified, these results will help in the identification and stratification of individuals who are at risk of IR and T2D for the purpose of prevention and treatment.
Keywords: AKT2; bioinformatics; insulin resistance; insulin signaling pathway; nsSNP; protein; type 2 diabetes.
Conflict of interest statement
The authors declare no conflict of interest exist.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

