Rheology of Gels and Yielding Liquids
- PMID: 37754396
- PMCID: PMC10529254
- DOI: 10.3390/gels9090715
Rheology of Gels and Yielding Liquids
Abstract
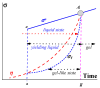
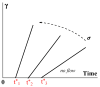
In this review, today's state of the art in the rheology of gels and transition through the yield stress of yielding liquids is discussed. Gels are understood as soft viscoelastic multicomponent solids that are in the incomplete phase separation state, which, under the action of external mechanical forces, do not transit into a fluid state but rupture like any solid material. Gels can "melt" (again, like any solids) due to a change in temperature or variation in the environment. In contrast to this type of rheology, yielding liquids (sometimes not rigorously referred to as "gels", especially in relation to colloids) can exist in a solid-like (gel-like) state and become fluid above some defined stress and time conditions (yield stress). At low stresses, their behavior is quite similar to that of permanent solid gels, including the frequency-independent storage modulus. The gel-to-sol transition considered in colloid chemistry is treated as a case of yielding. However, in many cases, the yield stress cannot be assumed to be a physical parameter since the solid-to-liquid transition happens in time and is associated with thixotropic effects. In this review, special attention is paid to various time effects. It is also stressed that plasticity is not equivalent to flow since (irreversible) plastic deformations are determined by stress but do not continue over time. We also discuss some typical errors, difficulties, and wrong interpretations of experimental data in studies of yielding liquids.
Keywords: flow; gel; gel-like state; yield stress; yielding liquids.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



















References
-
- de Gennes P.G. Soft matter (Nobel lecture) Angew. Chem. Int. Ed. Engl. 1992;31:842–845. doi: 10.1002/anie.199208421. - DOI
-
- de Souza Mendes P.R., Thompson R.L. Time-dependent yield stress materials. Curr. Opin. Colloid Interface Sci. 2019;43:15–25. doi: 10.1016/j.cocis.2019.01.018. - DOI
-
- de Souza Mendes P.R. Thixotropic elasto-viscoplastic model for structured fluids. Soft Matter. 2011;7:2471–2483. doi: 10.1039/c0sm01021a. - DOI
-
- de Souza Mandes P.R., Thomspon R.L. A unified approach to model elasto-viscoplastic thixotropic yield-stress materials and apparent yield-stress fluids. Rheol. Acta. 2013;52:673–694. doi: 10.1007/s00397-013-0699-1. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

