Quality by Design-Optimized Glycerosome-Enabled Nanosunscreen Gel of Rutin Hydrate
- PMID: 37754433
- PMCID: PMC10531150
- DOI: 10.3390/gels9090752
Quality by Design-Optimized Glycerosome-Enabled Nanosunscreen Gel of Rutin Hydrate
Abstract
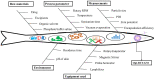
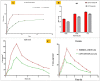
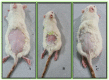
Sunburn is caused by prolonged exposure to ultraviolet (UV) rays from the sun, resulting in redness of the skin as well as tenderness, swelling, and blistering issues. During the healing process, it can cause peeling, irritation, and some long-term effects, including premature aging, pigmentation, and a high risk of skin cancer. Rutin has antioxidant and anti-inflammatory effects, which could potentially reduce inflammation and soothe sunburned skin. The objective of the current proposal is to develop and create carbopol gel-encased glycerosomes for the treatment of sunburn. The Design of Expert (DoE) technique was used to optimize the proposed formulation and was subjected to various characterization parameters such as nanovesicles size, polydispersity index (PDI), surface charge, entrapment efficiency (EE), and surface morphology. The optimized rutin-loaded glycerosomes (opt-RUT-loaded-GMs) were further characterised for drug release, 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay, and confocal laser scanning microscopy (CLSM). The formulation showed sustained release, greater permeation into the skin, and good antioxidant activity. The dermatokinetic study of opt-RUT-loaded-GMs confirms that the Rutin hydrate had better retention in the epidermis as compared to the dermis, owing to its potential for long lasting protection after topical application. It was observed that the prepared formulation was stable, highly safe, and had good sun protection factor (SPF) values that could be used as a suitable option for topical drug administration to maximize the therapeutic efficacy of the drugs.
Keywords: DoE; antioxidants; carbopol-gel; glycerosome; rutin; sunburn.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Advaita N., Lestari R.G., Aidid K.U., Sasongko H. In Vitro Evaluation of Sun Protection Factor of Vasconcellea Pubescens Fruit Extract. Int. Conf. Pharm. Res. Pract. 2015:122–125.
-
- Ahad A., Al-Saleh A.A., Al-Mohizea A.M., Al-Jenoobi F.I., Raish M., Yassin A.E.B., Alam M.A. Formulation and Characterization of Phospholipon 90 G and Tween 80 Based Transfersomes for Transdermal Delivery of Eprosartan Mesylate. Pharm. Dev. Technol. 2018;23:787–793. doi: 10.1080/10837450.2017.1330345. - DOI - PubMed
-
- Ali A., Aqil M., Imam S.S., Ahad A., Parveen A., Qadir A., Ali M.H., Akhtar M. Formulation and Evaluation of Embelin Loaded Nanoliposomes: Optimization, in Vitro and Ex Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2022;72:103414. doi: 10.1016/j.jddst.2022.103414. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

