Helicobacter pylori Status May Differentiate Two Distinct Pathways of Gastric Adenocarcinoma Carcinogenesis
- PMID: 37754493
- PMCID: PMC10527591
- DOI: 10.3390/curroncol30090578
Helicobacter pylori Status May Differentiate Two Distinct Pathways of Gastric Adenocarcinoma Carcinogenesis
Abstract
Background: We evaluated the phenotype of sporadic gastric cancer based on HP status and binding of a tumor risk marker monoclonal, Adnab-9.
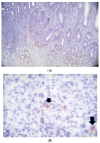
Methods: We compared a familial GC kindred with an extremely aggressive phenotype to HP-positive (HP+) and -negative (HP-) sporadic gastric adenocarcinoma (GC) patients in the same community to determine if similar phenotypes exist. This might facilitate gene discovery to understand the pathogenesis of aggressive GC phenotypes, particularly with publications implicating immune-related gene-based signatures, and the development of techniques to gauge the stance of the innate immune system (InImS), such as the FERAD ratio (blood ferritin:fecal Adnab-9 binding OD-background binding). Resection specimens for the sporadic and familial group were stained for HP and examined for intestinal metaplasia (IM) and immunostaining for Adnab-9. Familial kindred specimens were also tested for the E-cadherin mutation and APC (adenomatous polyposis coli). Survival was evaluated.
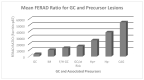
Results: Of 40 GC patients, 25% were HP+ with a greater proportion of intestinal metaplasia (IM) and gastric atrophy than the HP- group. The proband of the familial GC kindred, a 32-year-old mother with fatal GC, was survived by 13-year-old identical twins. Twin #1 was HP- with IM and Twin #2 was HP+. Both twins subsequently died of GC within two years. The twins did not have APC or E-cadherin mutations. The mean overall survival in the HP+ sporadic GC group was 2.47 ± 2.58 years and was 0.57 ± 0.60 years in the HP- group (p = 0.01). Survival in the kindred was 0.22 ± 0.24 years. Adnab-9 labeling was positive in fixed tissues of 50% of non-familial GC patients and in gastric tissue extract from Twin #2. The FERAD ratio was determined separately in six prospectively followed patient groups (n = 458) and was significantly lower in the gastric cancer patients (n = 10) and patients with stomach conditions predisposing them to GC (n = 214), compared to controls (n = 234 patients at increased risk for colorectal cancer but without cancer), suggesting a failure of the InImS.
Conclusion: The HP+ sporadic GC group appears to proceed through a sequence of HP infection, IM and atrophy before cancer supervenes, and the HP- phenotype appear to omit this sequence. The familial cases may represent a subset with both features, but the natural history strongly resembles that of the HP- group. Two different paths of carcinogenesis may exist locally for sporadic GC. The InImS may also be implicated in prognosis. Identifying these patients will allow for treatment stratification and early diagnosis to improve GC survival.
Keywords: Adnab-9; FERAD ratio; H. pylori; familial gastric cancer; monoclonal antibody.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The correlation between histological gastritis staging- 'OLGA/OLGIM' and serum pepsinogen test in assessment of gastric atrophy/intestinal metaplasia in China.Scand J Gastroenterol. 2017 Aug;52(8):822-827. doi: 10.1080/00365521.2017.1315739. Epub 2017 Apr 22. Scand J Gastroenterol. 2017. PMID: 28436254
-
Global DNA hypomethylation is an early event in Helicobacter pylori-related gastric carcinogenesis.J Clin Pathol. 2011 Aug;64(8):677-82. doi: 10.1136/jcp.2010.087858. Epub 2011 May 26. J Clin Pathol. 2011. PMID: 21617174
-
Overexpressions of RHOA, CSNK1A1, DVL2, FZD8, and LRP5 genes enhance gastric cancer development in the presence of Helicobacter pylori.Arab J Gastroenterol. 2023 May;24(2):91-97. doi: 10.1016/j.ajg.2023.01.004. Epub 2023 Jan 29. Arab J Gastroenterol. 2023. PMID: 36720664
-
Chemoprevention of gastric cancer by Helicobacter pylori eradication and its underlying mechanism.J Gastroenterol Hepatol. 2019 Aug;34(8):1287-1295. doi: 10.1111/jgh.14646. Epub 2019 Mar 27. J Gastroenterol Hepatol. 2019. PMID: 30828872 Review.
-
[Helicobacter pylori and gastric cancer. The incidence of infection in personal experience].Minerva Chir. 2002 Oct;57(5):649-55. Minerva Chir. 2002. PMID: 12370666 Review. Italian.
Cited by
-
The Non-Invasive Prediction of Colorectal Neoplasia (NIPCON) Study 1995-2022: A Comparison of Guaiac-Based Fecal Occult Blood Test (FOBT) and an Anti-Adenoma Antibody, Adnab-9.Int J Mol Sci. 2023 Dec 8;24(24):17257. doi: 10.3390/ijms242417257. Int J Mol Sci. 2023. PMID: 38139086 Free PMC article.
-
Predicting Regression of Barrett's Esophagus-Can All the King's Men Put It Together Again?Biomolecules. 2024 Sep 20;14(9):1182. doi: 10.3390/biom14091182. Biomolecules. 2024. PMID: 39334948 Free PMC article.
-
A Practical Format to Organize Cancer Constellations Using Innate Immune System Biomarkers: Implications for Early Diagnosis and Prognostication.Int J Transl Med (Basel). 2024 Dec 6;4(4):726-739. doi: 10.3390/ijtm4040050. Int J Transl Med (Basel). 2024. PMID: 40129890 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

