Efficacy and Safety of Anti-HER2 Targeted Therapy for Metastatic HR-Positive and HER2-Positive Breast Cancer: A Bayesian Network Meta-Analysis
- PMID: 37754530
- PMCID: PMC10528081
- DOI: 10.3390/curroncol30090615
Efficacy and Safety of Anti-HER2 Targeted Therapy for Metastatic HR-Positive and HER2-Positive Breast Cancer: A Bayesian Network Meta-Analysis
Abstract
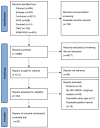
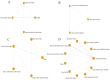
Despite the development of HER2-targeted drugs, achieving favorable outcomes for patients with HR+/HER2+MBC remains challenging. This study utilized Bayesian Network Meta-analysis to compare the efficacy and safety of anti-HER2 combination regimens. The primary analysis focused on progression-free survival (PFS), while secondary analyses included objective response rate, overall survival (OS) and the incidence rate of grade 3/4 adverse events (AEs). A comprehensive search across seven databases identified 25 randomized controlled trials for inclusion in this meta-analysis. For patients eligible for endocrinotherapy, our findings revealed that dual-target combined endocrine therapy, such as Her2-mAb+Her2-mAb+Endo (HR = 0.38; 95%CrI: 0.16-0.88) and Her2-mAb+Her2-tki+Endo (HR = 0.45; 95%CrI: 0.23-0.89), significantly improved PFS compared to endocrine therapy alone. According to the surface under the cumulative ranking curves (SUCRAs), Her2-mAb+Her2-mAb+Endo and Her2-mAb+Her2-tki+Endo ranked highest in terms of PFS and OS, respectively. For patients unsuitable for endocrine therapy, anti-HER2 dual-target combined chemotherapy, such as Her2-mAb+Her2-mAb+Chem (HR = 0.76; 95%CrI: 0.6-0.96) and Her2-mAb+Her2-tki+Chem (HR = 0.48; 95%CrI: 0.29-0.81), demonstrated significant improvements in PFS compared to Her2-mAb+Chem. The results were the same when compared with Her2-tki+Chem. According to the SUCRAs, Her2-mAb+Her2-tki+Chem and Her2-mAb+Her2-mAb+Chem ranked highest for PFS and OS, respectively. Subgroup analyses consistently supported these overall findings, indicating that dual-target therapy was the optimal approach irrespective of treatment line.
Keywords: Bayesian network meta-analysis; HER2-positive; HR-positive; metastatic breast cancer; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Efficacy of tucatinib for HER2-positive metastatic breast cancer after HER2-targeted therapy: a network meta-analysis.Future Oncol. 2021 Nov;17(33):4635-4647. doi: 10.2217/fon-2021-0742. Epub 2021 Aug 31. Future Oncol. 2021. PMID: 34463120
-
Phase III, Randomized Study of Dual Human Epidermal Growth Factor Receptor 2 (HER2) Blockade With Lapatinib Plus Trastuzumab in Combination With an Aromatase Inhibitor in Postmenopausal Women With HER2-Positive, Hormone Receptor-Positive Metastatic Breast Cancer: Updated Results of ALTERNATIVE.J Clin Oncol. 2021 Jan 1;39(1):79-89. doi: 10.1200/JCO.20.01894. Epub 2020 Aug 21. J Clin Oncol. 2021. PMID: 32822287 Clinical Trial.
-
Comparative efficacy, safety, and acceptability of single-agent poly (ADP-ribose) polymerase (PARP) inhibitors in BRCA-mutated HER2-negative metastatic or advanced breast cancer: a network meta-analysis.Aging (Albany NY). 2020 Nov 30;13(1):450-459. doi: 10.18632/aging.202152. Epub 2020 Nov 30. Aging (Albany NY). 2020. PMID: 33257598 Free PMC article.
-
A network meta-analysis of efficacy and safety for first-line and second/further-line therapies in postmenopausal women with hormone receptor-positive, HER2-negative, advanced breast cancer.BMC Med. 2024 Jan 12;22(1):13. doi: 10.1186/s12916-023-03238-2. BMC Med. 2024. PMID: 38212842 Free PMC article.
-
Cyclin-dependent kinase 4 and 6 inhibitors in hormone receptor-positive, human epidermal growth factor receptor-2 negative advanced breast cancer: a meta-analysis of randomized clinical trials.Breast Cancer Res Treat. 2020 Feb;180(1):21-32. doi: 10.1007/s10549-020-05528-2. Epub 2020 Jan 22. Breast Cancer Res Treat. 2020. PMID: 31970560 Review.
References
-
- Kaufman B., Mackey J.R., Clemens M.R., Bapsy P.P., Vaid A., Wardley A., Tjulandin S., Jahn M., Lehle M., Feyereislova A., et al. Trastuzumab Plus Anastrozole Versus Anastrozole Alone for the Treatment of Postmenopausal Women With Human Epidermal Growth Factor Receptor 2–Positive, Hormone Receptor–Positive Metastatic Breast Cancer: Results From the Randomized Phase III TAnDEM Study. J. Clin. Oncol. 2009;27:5529–5537. doi: 10.1200/JCO.2008.20.6847. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

