Capecitabine/Mitomycin versus 5-Fluorouracil/Mitomycin in Combination with Simultaneous Integrated Boost Intensity-Modulated Radiation Therapy for Anal Cancer
- PMID: 37754536
- PMCID: PMC10528380
- DOI: 10.3390/curroncol30090621
Capecitabine/Mitomycin versus 5-Fluorouracil/Mitomycin in Combination with Simultaneous Integrated Boost Intensity-Modulated Radiation Therapy for Anal Cancer
Abstract
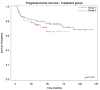
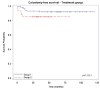
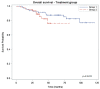
Since EXTRA, a non-randomized phase II trial with 31 patients, explored the use of capecitabine, mitomycin and radiation therapy (RT) in the treatment of localized squamous cell carcinoma of the anal canal (SCCAC), this treatment has been considered as an acceptable alternative to infusional 5-FU. However, the differences in efficacy between capecitabine and 5-FU in chemoradiation therapy (CRT) with simultaneous integrated boost (SIB) radiation therapy (SIB-IMRT) for local SCCAC are not well documented. Patients included in this prospective monocentric cohort study were treated with SIB-RapidArc (a unique RT method treatment for all patients: identical technique, volume and constraints for at-risk organs), mitomycin C and 5-FU each day of RT for 7 weeks (group 1) or capecitabine each day of RT (group 2). Patients treated between July 2009 and August 2017 (group 1) and between November 2012 and April 2018 (group 2) for local SCCAC T2-4 classified as N, M0 or T, N1-3, M0 were included. Primary endpoints were progression-free survival (PFS) and acute toxicities. Results: One hundred forty-seven patients were included, 91 in group 1 and 56 in group 2. The two groups were statistically comparable in terms of sex, Eastern Cooperative Oncology Group Performance Status (ECOG PS) and TNM. With a median duration of follow-up of 53.5 months, the PFS rate at 3 years was 80% for group 1 and 75% for group 2 (p = 0.32). The 3-year colostomy-free survival rate was 92% for group 1 and 85% for group 2 (p = 0.11). The rate of patients with at least one grade 3 or higher acute toxicity was 35.5% in group 1 and 21.4% in group 2 (p = 0.10), with a trend of fewer acute toxicities with capecitabine. Conclusion: Capecitabine/mitomycin in combination with SIB RapidArc radiation therapy for anal cancer seems as effective as 5-FU-based chemotherapy and is well tolerated with minimal toxicity.
Keywords: 5-FU; anal cancer; capecitabin; radiation therapy; toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Bouvier A.M., Belot A., Manfredi S., Jooste V., Uhry Z., Faivre J., Duport N., Grabar S., French Network of Cancer Registries FRANCIM Trends of incidence and survival in squamous-cell carcinoma of the anal canal in France: A population-based study. Eur. J. Cancer. Prev. 2016;25:182–187. doi: 10.1097/CEJ.0000000000000163. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

