Association of magnetic resonance imaging phenotypes and serum biomarker levels with treatment response and long-term disease outcomes in multiple sclerosis patients
- PMID: 37754568
- PMCID: PMC11235849
- DOI: 10.1111/ene.16077
Association of magnetic resonance imaging phenotypes and serum biomarker levels with treatment response and long-term disease outcomes in multiple sclerosis patients
Abstract
Background and purpose: The aim was to evaluate whether magnetic resonance imaging (MRI) phenotypes defined by inflammation and neurodegeneration markers correlate with serum levels of neurofilament light chain (NfL) and glial fibrillary acidic protein (GFAP) in relapsing-remitting multiple sclerosis (RRMS) patients; and to explore the role of radiological phenotypes and biomarker levels on treatment response and long-term prognostic outcomes.
Methods: Magnetic resonance imaging scans from 80 RRMS patients were classified at baseline of interferon-beta (IFNβ) treatment into radiological phenotypes defined by high and low inflammation and high and low neurodegeneration, based on the number of contrast-enhancing lesions, brain parenchymal fraction and the relative volume of non-enhancing black holes on T1-weighted images. Serum levels of NfL and GFAP were measured at baseline with single molecule array (Simoa) assays. MRI phenotypes and serum biomarker levels were investigated for their association with IFNβ response, and times to second-line therapies, secondary-progressive MS (SPMS) conversion and Expanded Disability Status Scale (EDSS) 6.0.
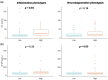
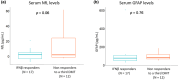
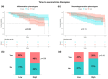
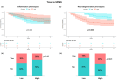
Results: Mean (SD) follow-up was 17 (2.9) years. Serum NfL levels and GFAP were higher in the high inflammation (p = 0.04) and high neurodegeneration phenotypes (p = 0.03), respectively. The high inflammation phenotype was associated with poor response to IFNβ treatment (p = 0.04) and with shorter time to second-line therapies (p = 0.04). In contrast, the high neurodegeneration phenotype was associated with shorter time to SPMS (p = 0.006) and a trend towards shorter time to EDSS 6.0 (p = 0.09). High serum NfL levels were associated with poor response to IFNβ treatment (p = 0.004).
Conclusions: Magnetic resonance imaging phenotypes defined by inflammation and neurodegeneration correlate with serum biomarker levels, and both have prognostic implications in treatment response and long-term disease outcomes.
Keywords: long-term prognosis; magnetic resonance imaging; multiple sclerosis; neurofilament light chain; treatment response.
© 2023 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
Luciana Midaglia has nothing to disclose. Alex Rovira serves on scientific advisory boards for Novartis, Sanofi‐Genzyme, Synthetic MRI, Roche, Biogen, TensorMedical, Bristol Myers and OLEA Medical, and has received speaker honoraria from Sanofi‐Genzyme, Merck‐Serono, Bayer, Teva Pharmaceutical Industries Ltd, Novartis, Roche and Biogen. Berta Miró has nothing to disclose. Jordi Río has received speaking honoraria and personal compensation for participating on advisory boards from Biogen‐Idec, Genzyme, Merck‐Serono, Mylan, Novartis, Roche, Teca and Sanofi‐Aventis. Nicolás Fissolo has nothing to disclose. Joaquín Castilló has nothing to disclose. Alex Sánchez has nothing to disclose. Xavier Montalban has received speaking honoraria and travel expenses for participation in scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past with Actelion, Amirall, Bayer, Biogen, Celgene, Genzyme, Hoffmann‐La Roche, Novartis, Oryzon Genomics, Sanofi‐Genzyme and Teva Pharmaceutical. Manuel Comabella has received compensation for consulting services and speaking honoraria from Bayer Schering Pharma, Merk Serono, Biogen‐Idec, Teva Pharmaceuticals, Sanofi‐Aventis and Novartis.
Figures


Similar articles
-
Baseline serum neurofilament light chain levels differentiate aggressive from benign forms of relapsing-remitting multiple sclerosis: a 20-year follow-up cohort.J Neurol. 2024 Apr;271(4):1599-1609. doi: 10.1007/s00415-023-12135-w. Epub 2023 Dec 12. J Neurol. 2024. PMID: 38085343 Free PMC article.
-
Glial and neuroaxonal biomarkers in a multiple sclerosis (MS) cohort.Hell J Nucl Med. 2019 Sep-Dec;22 Suppl 2:113-121. Hell J Nucl Med. 2019. PMID: 31802051
-
Early neurofilament light and glial fibrillary acidic protein levels improve predictive models of multiple sclerosis outcomes.Mult Scler Relat Disord. 2023 Jun;74:104695. doi: 10.1016/j.msard.2023.104695. Epub 2023 Apr 2. Mult Scler Relat Disord. 2023. PMID: 37060852
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
-
Management of worsening multiple sclerosis with mitoxantrone: a review.Clin Ther. 2006 Apr;28(4):461-74. doi: 10.1016/j.clinthera.2006.04.013. Clin Ther. 2006. PMID: 16750460 Review.
Cited by
-
[XVI Post-ECTRIMS Meeting: review of the new developments presented at the 2023 ECTRIMS Congress (I)].Rev Neurol. 2024 Jul 1;79(1):21-29. doi: 10.33588/rn.7901.2024170. Rev Neurol. 2024. PMID: 38934946 Free PMC article. Review. Spanish.
-
The Role of Glial Fibrillary Acidic Protein as a Biomarker in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder: A Systematic Review and Meta-Analysis.Medicina (Kaunas). 2024 Jun 26;60(7):1050. doi: 10.3390/medicina60071050. Medicina (Kaunas). 2024. PMID: 39064479 Free PMC article.
-
Cerebrospinal fluid levels of chitinase 3-like 1 and interleukin-6 can predict response to platform therapies in relapsing multiple sclerosis.J Neurol. 2025 Aug 25;272(9):592. doi: 10.1007/s00415-025-13321-8. J Neurol. 2025. PMID: 40855223
References
-
- Oh J, Vidal‐Jordana A, Montalban X. Multiple sclerosis: clinical aspects. Curr Opin Neurol. 2018;31:752‐759. - PubMed
-
- Gafson A, Craner MJ, Matthews PM. Personalised medicine for multiple sclerosis care. Mult Scler. 2017;23:362‐369. - PubMed
-
- Dobson R, Giovannoni G. Multiple sclerosis—a review. Eur J Neurol. 2019;26:27‐40. - PubMed
-
- Midaglia L, Sastre‐Garriga J, Montalban X. Clinical monitoring of multiple sclerosis patients by means of digital technology, a field in the midst of a revolution. Rev Neurol. 2021;73:210‐218. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

