Streptomyces albidoflavus Q antifungal metabolites inhibit the ergosterol biosynthesis pathway and yeast growth in fluconazole-resistant Candida glabrata: phylogenomic and metabolomic analyses
- PMID: 37754674
- PMCID: PMC10581079
- DOI: 10.1128/spectrum.01271-23
Streptomyces albidoflavus Q antifungal metabolites inhibit the ergosterol biosynthesis pathway and yeast growth in fluconazole-resistant Candida glabrata: phylogenomic and metabolomic analyses
Abstract
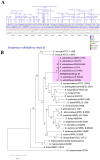
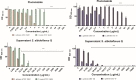
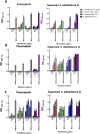
There is an urgent need to develop new antifungals due to the increasing prevalence of multidrug-resistant fungal infections and the recent emergence of COVID-19-associated candidiasis. A good study model for evaluating new antifungal compounds is Candida glabrata, an opportunistic fungal pathogen with intrinsic resistance to azoles (the most common clinical drugs for treating fungal infections). The aim of the current contribution was to conduct in vitro tests of antifungal metabolites produced by the bacteria Streptomyces albidoflavus Q, identify their molecular structures, and utilize several techniques to provide evidence of their therapeutic target. S. albidoflavus was isolated from maize rhizospheric soil in Mexico and identified by phylogenomic analysis using a 92-gene core. Of the 66 metabolites identified in S. albidoflavus Q by a liquid chromatography-high resolution mass spectrometry (LC-HRMS) metabolomic analysis of the lyophilized supernatant, six were selected by the Way2drug server based on their in silico binding to the likely target, 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR, the key enzyme in the ergosterol biosynthesis pathway). Molecular modeling studies show a relatively high binding affinity for the CgHMGR enzyme by two secondary metabolites: isogingerenone B (diaryl heptanoid) and notoginsenoside J (polycyclic triterpene). These secondary metabolites were able to inhibit ergosterol synthesis and affect yeast viability in vitro. They also caused alterations in the ultrastructure of the yeast cytoplasmic membrane, as evidenced by transmission electron microscopy. The putative target of isogingerenone B and notoginsenoside J is distinct from that of azole drugs (the most common clinical antifungals). The target for the latter is the lanosterol 14 alpha-demethylase enzyme (Erg11). IMPORTANCE Multidrug resistance has emerged among yeasts of the genus Candida, posing a severe threat to global health. The problem has been exacerbated by the pandemic associated with COVID-19, during which resistant strains of Candida auris and Candida glabrata have been isolated from patients infected with the SARS-CoV-2 virus. To confront this challenge, the World Health Organization has invoked scientists to search for new antifungals with alternative molecular targets. This study identified 66 metabolites produced by the bacteria Streptomyces albidoflavus Q, 6 of which had promising properties for potential antifungal activity. The metabolites were tested in vitro as inhibitors of ergosterol synthesis and C. glabrata growth, with positive results. They were also found to damage the cytoplasmic membrane of the fungus. The corresponding molecular structures and their probable therapeutic target were established. The target is apparently distinct from that of azole drugs.
Keywords: Candida glabrata; HMGR (EC 1.1.1.34); Streptomyces albidoflavus; WGS; actinobacteria; actinomycete; antifungal; cytoplasmic membrane; ergosterol; metabolomics; multi-drug resistance; plant-associated metabolites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

