Cesin, a short natural variant of nisin, displays potent antimicrobial activity against major pathogens despite lacking two C-terminal macrocycles
- PMID: 37754751
- PMCID: PMC10581189
- DOI: 10.1128/spectrum.05319-22
Cesin, a short natural variant of nisin, displays potent antimicrobial activity against major pathogens despite lacking two C-terminal macrocycles
Abstract
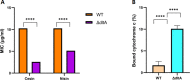
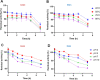
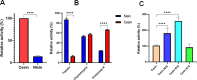
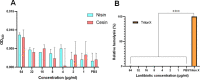
Nisin is a widely used lantibiotic owing to its potent antimicrobial activity and its food-grade status. Its mode of action includes cell wall synthesis inhibition and pore formation, which are attributed to the lipid II binding and pore-forming domains, respectively. We discovered cesin, a short natural variant of nisin, produced by the psychrophilic anaerobe Clostridium estertheticum. Unlike other natural nisin variants, cesin lacks the two terminal macrocycles constituting the pore-forming domain. The current study aimed at heterologous expression and characterization of the antimicrobial activity and physicochemical properties of cesin. Following the successful heterologous expression of cesin in Lactococcus lactis, the lantibiotic demonstrated a broad and potent antimicrobial profile comparable to that of nisin. Determination of its mode of action using lipid II and lipoteichoic acid binding assays linked the potent antimicrobial activity to lipid II binding and electrostatic interactions with teichoic acids. Fluorescence microscopy showed that cesin lacks pore-forming ability in its natural form. Stability tests have shown the lantibiotic is highly stable at different pH values and temperature conditions, but that it can be degraded by trypsin. However, a bioengineered analog, cesin R15G, overcame the trypsin degradation, while keeping full antimicrobial activity. This study shows that cesin is a novel (small) nisin variant that efficiently kills target bacteria by inhibiting cell wall synthesis without pore formation. IMPORTANCE The current increase in antibiotic-resistant pathogens necessitates the discovery and application of novel antimicrobials. In this regard, we recently discovered cesin, which is a short natural variant of nisin produced by the psychrophilic Clostridium estertheticum. However, its suitability as an antimicrobial compound was in doubt due to its structural resemblance to nisin(1-22), a bioengineered short variant of nisin with low antimicrobial activity. Here, we show by heterologous expression, purification, and characterization that the potency of cesin is not only much higher than that of nisin(1-22), but that it is even comparable to the full-length nisin, despite lacking two C-terminal rings that are essential for nisin's activity. We show that cesin is a suitable scaffold for bioengineering to improve its applicability, such as resistance to trypsin. This study demonstrates the suitability of cesin for future application in food and/or for health as a potent and stable antimicrobial compound.
Keywords: Clostridium estertheticum; bacteriocin; cesin; lipid II; nisin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources

