Biological Performance of Duplex PEO + CNT/PCL Coating on AZ31B Mg Alloy for Orthopedic and Dental Applications
- PMID: 37754889
- PMCID: PMC10532417
- DOI: 10.3390/jfb14090475
Biological Performance of Duplex PEO + CNT/PCL Coating on AZ31B Mg Alloy for Orthopedic and Dental Applications
Abstract
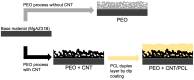
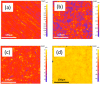
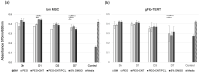
To regulate the degradation rate and improve the surface biocompatibility of the AZ31B magnesium alloy, three different coating systems were produced via plasma electrolytic oxidation (PEO): simple PEO, PEO incorporating multi-walled carbon nanotubes (PEO + CNT), and a duplex coating that included a polycaprolactone top layer (PEO + CNT/PCL). Surfaces were characterized by chemical content, roughness, topography, and wettability. Biological properties analysis included cell metabolism and adhesion. PEO ± CNT resulted in an augmented surface roughness compared with the base material (BM), while PCL deposition produced the smoothest surface. All surfaces had a contact angle below 90°. The exposure of gFib-TERT and bmMSC to culture media collected after 3 or 24 h did not affect their metabolism. A decrease in metabolic activity of 9% and 14% for bmMSC and of 14% and 29% for gFib-TERT was observed after 3 and 7 days, respectively. All cells died after 7 days of exposure to BM and after 15 days of exposure to coated surfaces. Saos-2 and gFib-TERT adhered poorly to BM, in contrast to bmMSC. All cells on PEO anchored into the pores with filopodia, exhibited tiny adhesion protrusions on PEO + CNT, and presented a web-like spreading with lamellipodia on PEO + CNT/PCL. The smooth and homogenous surface of the duplex PEO + CNT/PCL coating decreased magnesium corrosion and led to better biological functionality.
Keywords: biodegradable Mg-based implant; cell adhesion; cell metabolism; multi-walled carbon nanotubes (MWCNT); plasma electrolyte oxidation (PEO); polycaprolactone (PCL).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Geetha M., Singh A.K., Asokamani R., Gogia A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog. Mater. Sci. 2009;54:397–425. doi: 10.1016/j.pmatsci.2008.06.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

