Small Cationic Cysteine-Rich Defensin-Derived Antifungal Peptide Controls White Mold in Soybean
- PMID: 37754982
- PMCID: PMC10532163
- DOI: 10.3390/jof9090873
Small Cationic Cysteine-Rich Defensin-Derived Antifungal Peptide Controls White Mold in Soybean
Abstract
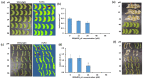
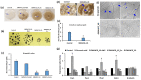
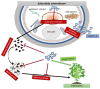
White mold disease caused by a necrotrophic ascomycete pathogen Sclerotinia sclerotiorum results in serious economic losses of soybean yield in the USA. Lack of effective genetic resistance to this disease in soybean germplasm and increasing pathogen resistance to fungicides makes white mold difficult to manage. Small cysteine-rich antifungal peptides with multi-faceted modes of action possess potential for development as sustainable spray-on bio-fungicides. We have previously reported that GMA4CG_V6 peptide, a 17-amino acid variant of the MtDef4 defensin-derived peptide GMA4CG containing the active γ-core motif, exhibits potent antifungal activity against the gray mold fungal pathogen Botrytis cinerea in vitro and in planta. GMA4CG_V6 exhibited antifungal activity against an aggressive field isolate of S. sclerotiorum 555 in vitro with an MIC value of 24 µM. At this concentration, internalization of this peptide into fungal cells occurred prior to discernible membrane permeabilization. GMA4CG_V6 markedly reduced white mold disease symptoms when applied to detached soybean leaves, pods, and stems. Its spray application on soybean plants provided robust control of this disease. GMA4CG_V6 at sub-lethal concentrations reduced sclerotia production. It was also non-phytotoxic to soybean plants. Our results demonstrate that GMA4CG_V6 peptide has potential for development as a bio-fungicide for white mold control in soybean.
Keywords: Sclerotinia sclerotiorum; antifungal peptide; bio-fungicide; modes of action; soybean.
Conflict of interest statement
The authors declare no conflict of interest. The funding agency played no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Shahbandeh M. Production Value of Soybeans in the U.S. 2000–2021. 2022. [(accessed on 25 April 2023)]. Available online: www.statista.com/statistics/192071/production-value-of-soybeans-for-bean....
-
- Allen T.W., Bradley C.A., Sisson A.J., Byamukama E., Chilvers M.I., Coker C.M. Soybean yield loss estimates due to diseases in the United States and Ontario, Canada from 2010 to 2014. Plant Health Prog. 2017;18:19–27. doi: 10.1094/PHP-RS-16-0066. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

