Antibacterial Polyketides Isolated from the Marine-Derived Fungus Fusarium solani 8388
- PMID: 37754983
- PMCID: PMC10532693
- DOI: 10.3390/jof9090875
Antibacterial Polyketides Isolated from the Marine-Derived Fungus Fusarium solani 8388
Abstract
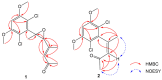
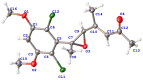
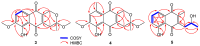
Seven new polyketides named fusarisolins F-K (1-6) and fusarin I (7) were isolated from the marine-derived fungus Fusarium solani 8388, together with the known anhydrojavanicin (8), 5-deoxybostry coidin (9), and scytalol A (10). Their structures were established by comprehensive spectroscopic data analyses, and by comparison of the 1H and 13C NMR data with those reported in literature. Fusarisolin F (1) contained both a dichlorobenzene group and an ethylene oxide unit, which was rare in nature. In the bioassays, fusarisolin I (4), fusarisolin J (5), and 5-deoxybostry coidin (9) exhibited obvious antibacterial activities against methicillin-resistant Staphylococcus aureus n315 with MIC values of 3, 3, and 6 μg/mL, respectively. Fusarisolin H (3) and fusarisolin J (5) showed inhibitory effects against methicillin-resistant Staphylococcus aureus NCTC 10442 with the same MIC value of 6 μg/mL. With the exception of 5, all other compounds did not show or showed weak cytotoxicities against HeLa, A549, and KB cells; while fusarisolin J (5) demonstrated moderate cytotoxicities against the three human cancer cell lines with CC50 values between 9.21 and 14.02 μM.
Keywords: Fusarium solani; antibacterial; cytotoxicity; fungus; marine microorganism; polyketide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Manning B.B., Abbas H.K. The effect of Fusarium mycotoxins deoxynivalenol, fumonisin, and moniliformin from contaminated moldy grains on aquaculture fish. Toxin Rev. 2012;31:11–15. doi: 10.3109/15569543.2011.651519. - DOI
-
- Antonissen G., Martel A., Pasmans F., Ducatelle R., Verbrugghe E., Vandenbroucke V., Li S.J., Haesebrouck F., Van Immerseel F., Croubels S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins. 2014;6:430–452. doi: 10.3390/toxins6020430. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

