Axenic Long-Term Cultivation of Pneumocystis jirovecii
- PMID: 37755011
- PMCID: PMC10533121
- DOI: 10.3390/jof9090903
Axenic Long-Term Cultivation of Pneumocystis jirovecii
Abstract
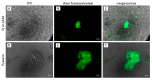
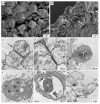
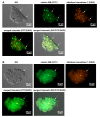
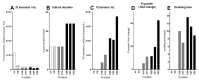
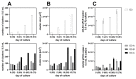
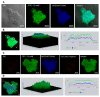
Pneumocystis jirovecii, a fungus causing severe Pneumocystis pneumonia (PCP) in humans, has long been described as non-culturable. Only isolated short-term experiments with P. jirovecii and a small number of experiments involving animal-derived Pneumocystis species have been published to date. However, P. jirovecii culture conditions may differ significantly from those of animal-derived Pneumocystis, as there are major genotypic and phenotypic differences between them. Establishing a well-performing P. jirovecii cultivation is crucial to understanding PCP and its pathophysiological processes. The aim of this study, therefore, was to develop an axenic culture for Pneumocystis jirovecii. To identify promising approaches for cultivation, a literature survey encompassing animal-derived Pneumocystis cultures was carried out. The variables identified, such as incubation time, pH value, vitamins, amino acids, and other components, were trialed and adjusted to find the optimum conditions for P. jirovecii culture. This allowed us to develop a medium that produced a 42.6-fold increase in P. jirovecii qPCR copy numbers after a 48-day culture. Growth was confirmed microscopically by the increasing number and size of actively growing Pneumocystis clusters in the final medium, DMEM-O3. P. jirovecii doubling time was 8.9 days (range 6.9 to 13.6 days). In conclusion, we successfully cultivated P. jirovecii under optimized cell-free conditions in a 70-day long-term culture for the first time. However, further optimization of the culture conditions for this slow grower is indispensable.
Keywords: A549; DMEM; Pneumocystis; Pneumocystis jirovecii; axenic; culture; human lung carcinoma cells.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- Burghi G., Biard L., Roux A., Valade S., Robert-Gangneux F., Hamane S., Maubon D., Debourgogne A., Le Gal S., Dalle F., et al. Characteristics and outcome according to underlying disease in non-AIDS patients with acute respiratory failure due to Pneumocystis pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:1191–1198. doi: 10.1007/s10096-020-04118-w. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

