Kocuria flava, a Bacterial Endophyte of the Marine Macroalga Bryopsis plumosa, Emits 8-Nonenoic Acid Which Inhibits the Aquaculture Pathogen Saprolegnia parasitica
- PMID: 37755090
- PMCID: PMC10532832
- DOI: 10.3390/md21090476
Kocuria flava, a Bacterial Endophyte of the Marine Macroalga Bryopsis plumosa, Emits 8-Nonenoic Acid Which Inhibits the Aquaculture Pathogen Saprolegnia parasitica
Abstract
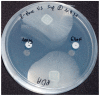
Secondary metabolites-organic compounds that are often bioactive-produced by endophytes, among others, provide a selective advantage by increasing the organism's survivability. Secondary metabolites mediate the symbiotic relationship between endophytes and their host, potentially providing the host with tolerance to, and protection against biotic and abiotic stressors. Secondary metabolites can be secreted as a dissolved substance or emitted as a volatile. In a previous study, we isolated bioactive endophytes from several macroalgae and tested them in vitro for their ability to inhibit major disease-causing pathogens of aquatic animals in the aquaculture industry. One endophyte (isolate Abp5, K. flava) inhibited and killed, in vitro, the pathogen Saprolegnia parasitica, an oomycete that causes saprolegniasis-a disease affecting a wide range of aquatic animals. Here, using analytical chemistry tools, we found that Abp5 produces the volatile organic compound (VOC) 8-nonenoic acid. Once we confirmed the production of this compound by the endophyte, we tested the compound's ability to treat S. parasitica in in vitro and in vivo bioassays. In the latter, we found that 5 mg/L of the compound improves the survival of larvae challenged with S. parasitica by 54.5%. Our isolation and characterization of the VOC emitted by the endophytic K. flava establish the groundwork for future studies of endophytic biocontrol agents from macroalgae. Use of this compound could enable managing oomycete agricultural pathogens in general, and S. parasitica in particular, a major causal agent in aquaculture diseases.
Keywords: 8-nonenoic acid; aquaculture; disease management; endophyte; macroalga; secondary metabolite.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





Similar articles
-
Quantitative proteomic analysis of plasma membranes from the fish pathogen Saprolegnia parasitica reveals promising targets for disease control.Microbiol Spectr. 2024 Aug 6;12(8):e0034824. doi: 10.1128/spectrum.00348-24. Epub 2024 Jun 18. Microbiol Spectr. 2024. PMID: 38888349 Free PMC article.
-
Linalool combats Saprolegnia parasitica infections through direct killing of microbes and modulation of host immune system.Elife. 2025 Apr 4;13:RP100393. doi: 10.7554/eLife.100393. Elife. 2025. PMID: 40183210 Free PMC article.
-
New Approaches for Controlling Saprolegnia parasitica, the Causal Agent of a Devastating Fish Disease.Trop Life Sci Res. 2014 Dec;25(2):101-9. Trop Life Sci Res. 2014. PMID: 27073602 Free PMC article.
-
Current Scenario and Future Prospects of Endophytic Microbes: Promising Candidates for Abiotic and Biotic Stress Management for Agricultural and Environmental Sustainability.Microb Ecol. 2023 Oct;86(3):1455-1486. doi: 10.1007/s00248-023-02190-1. Epub 2023 Mar 14. Microb Ecol. 2023. PMID: 36917283 Free PMC article. Review.
-
Metabolomic Insights Into Endophyte-Derived Bioactive Compounds.Front Microbiol. 2022 Mar 2;13:835931. doi: 10.3389/fmicb.2022.835931. eCollection 2022. Front Microbiol. 2022. PMID: 35308367 Free PMC article. Review.
Cited by
-
Carotenoid pigments of Kocuria flava PUTS1_3 isolated from sediments of Puttalam lagoon mangrove ecosystem, Sri Lanka exhibit bioactive properties.Sci Rep. 2025 Apr 30;15(1):15226. doi: 10.1038/s41598-025-93643-9. Sci Rep. 2025. PMID: 40307338 Free PMC article.
References
-
- Bacon C.W., White J.F. Functions, mechanisms and regulation of endophytic and epiphytic microbial communities of plants. Symbiosis. 2016;68:87–98. doi: 10.1007/s13199-015-0350-2. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

