Krill Oil's Protective Benefits against Ultraviolet B-Induced Skin Photoaging in Hairless Mice and In Vitro Experiments
- PMID: 37755092
- PMCID: PMC10533088
- DOI: 10.3390/md21090479
Krill Oil's Protective Benefits against Ultraviolet B-Induced Skin Photoaging in Hairless Mice and In Vitro Experiments
Abstract
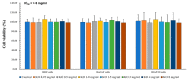
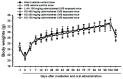
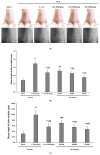
Krill oil (KO) shows promise as a natural marine-derived ingredient for improving skin health. This study investigated its antioxidant, anti-inflammatory, anti-wrinkle, and moisturizing effects on skin cells and UVB-induced skin photoaging in hairless mice. In vitro assays on HDF, HaCaT, and B16/F10 cells, as well as in vivo experiments on 60 hairless mice were conducted. A cell viability assay, diphenyl-1-picryhydrazyl (DPPH) radical scavenging activity test, elastase inhibition assay, procollagen content test, MMP-1 inhibition test, and hyaluronan production assay were used to experiment on in vitro cell models. Mice received oral KO administration (100, 200, or 400 mg/kg) once a day for 15 weeks and UVB radiation three times a week. L-Ascorbic acid (L-AA) was orally administered at 100 mg/kg once daily for 15 weeks, starting from the initial ultraviolet B (UVB) exposures. L-AA administration followed each UVB session (0.18 J/cm2) after one hour. In vitro, KO significantly countered UVB-induced oxidative stress, reduced wrinkles, and prevented skin water loss by enhancing collagen and hyaluronic synthesis. In vivo, all KO dosages showed dose-dependent inhibition of oxidative stress-induced inflammatory photoaging-related skin changes. Skin mRNA expressions for hyaluronan synthesis and collagen synthesis genes also increased dose-dependently after KO treatment. Histopathological analysis confirmed that krill oil (KO) ameliorated the damage caused by UVB-irradiated skin tissues. The results imply that KO could potentially act as a positive measure in diminishing UVB-triggered skin photoaging and address various skin issues like wrinkles and moisturization when taken as a dietary supplement.
Keywords: krill oil; marine-derived ingredients; skin photoaging; ultraviolet.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
Wheat Extract Oil (WEO) Attenuates UVB-Induced Photoaging via Collagen Synthesis in Human Keratinocytes and Hairless Mice.Nutrients. 2020 Jan 22;12(2):300. doi: 10.3390/nu12020300. Nutrients. 2020. PMID: 31979027 Free PMC article.
-
Conditioned medium from human bone marrow-derived mesenchymal stem cells promotes skin moisturization and effacement of wrinkles in UVB-irradiated SKH-1 hairless mice.Photodermatol Photoimmunol Photomed. 2016 May;32(3):120-8. doi: 10.1111/phpp.12224. Epub 2015 Dec 15. Photodermatol Photoimmunol Photomed. 2016. PMID: 26577060
-
Anti-wrinkling effects of the mixture of vitamin C, vitamin E, pycnogenol and evening primrose oil, and molecular mechanisms on hairless mouse skin caused by chronic ultraviolet B irradiation.Photodermatol Photoimmunol Photomed. 2007 Oct;23(5):155-62. doi: 10.1111/j.1600-0781.2007.00298.x. Photodermatol Photoimmunol Photomed. 2007. PMID: 17803593
-
Hydrogel-Based Therapies for Photoaging: Current Advances and Future Perspectives.J Cosmet Dermatol. 2025 Jun;24(6):e70295. doi: 10.1111/jocd.70295. J Cosmet Dermatol. 2025. PMID: 40515408 Free PMC article. Review.
-
Mitigating Glycation and Oxidative Stress in Aesthetic Medicine: Hyaluronic Acid and Trehalose Synergy for Anti-AGEs Action in Skin Aging Treatment.Clin Cosmet Investig Dermatol. 2024 Nov 28;17:2701-2712. doi: 10.2147/CCID.S476362. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 39624066 Free PMC article. Review.
Cited by
-
Development of topical silver nano gel formulation of Bixin: Characterization, and evaluation of anticancer activity.Saudi Pharm J. 2024 Jul;32(7):102125. doi: 10.1016/j.jsps.2024.102125. Epub 2024 Jun 2. Saudi Pharm J. 2024. PMID: 38933714 Free PMC article.
-
Vorinostat attenuates UVB-induced skin senescence by modulating NF-κB and mTOR signaling pathways.Sci Rep. 2025 Mar 29;15(1):10905. doi: 10.1038/s41598-025-95624-4. Sci Rep. 2025. PMID: 40158057 Free PMC article.
-
Krill oil: nutraceutical potential in skin health and disease.Front Nutr. 2024 Jul 12;11:1388155. doi: 10.3389/fnut.2024.1388155. eCollection 2024. Front Nutr. 2024. PMID: 39070257 Free PMC article. Review.
-
Effects of supplementation with krill oil on blood parameters, hair quality, and fecal microbiota in male beagle dogs.Front Microbiol. 2025 Aug 1;16:1587149. doi: 10.3389/fmicb.2025.1587149. eCollection 2025. Front Microbiol. 2025. PMID: 40822403 Free PMC article.
-
Matrix Metalloproteinases on Skin Photoaging.J Cosmet Dermatol. 2024 Dec;23(12):3847-3862. doi: 10.1111/jocd.16558. Epub 2024 Sep 4. J Cosmet Dermatol. 2024. PMID: 39230065 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

