Bacterial Outer Membrane Vesicles and Immune Modulation of the Host
- PMID: 37755174
- PMCID: PMC10536716
- DOI: 10.3390/membranes13090752
Bacterial Outer Membrane Vesicles and Immune Modulation of the Host
Abstract
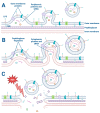
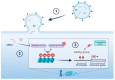
This article reviews the role of outer membrane vesicles (OMVs) in mediating the interaction between Gram-negative bacteria and their human hosts. OMVs are produced by a diverse range of Gram-negative bacteria during infection and play a critical role in facilitating host-pathogen interactions without requiring direct cell-to-cell contact. This article describes the mechanisms by which OMVs are formed and subsequently interact with host cells, leading to the transport of microbial protein virulence factors and short interfering RNAs (sRNA) to their host targets, exerting their immunomodulatory effects by targeting specific host signaling pathways. Specifically, this review highlights mechanisms by which OMVs facilitate chronic infection through epigenetic modification of the host immune response. Finally, this review identifies critical knowledge gaps in the field and offers potential avenues for future OMV research, specifically regarding rigor and reproducibility in OMV isolation and characterization methods.
Keywords: DNA methylation (DNAm); immune modulation; inter-kingdom communication; outer membrane vesicles (OMVs).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Yáñez-Mó M., Siljander P.R.M., Andreu Z., Zavec A.B., Borràs F.E., Buzas E.I., Buzas K., Casal E., Cappello F., Carvalho J., et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. - DOI - PMC - PubMed
-
- Koeppen K., Hampton T.H., Jarek M., Scharfe M., Gerber S.A., Mielcarz D.W., Demers E.G., Dolben E.L., Hammond J.H., Hogan D.A., et al. A Novel Mechanism of Host-Pathogen Interaction through SRNA in Bacterial Outer Membrane Vesicles. PLoS Pathog. 2016;12:e1005672. doi: 10.1371/journal.ppat.1005672. - DOI - PMC - PubMed
-
- Kikuchi Y., Obana N., Toyofuku M., Kodera N., Soma T., Ando T., Fukumori Y., Nomura N., Taoka A. Diversity of Physical Properties of Bacterial Extracellular Membrane Vesicles Revealed through Atomic Force Microscopy Phase Imaging. Nanoscale. 2020;12:7950–7959. doi: 10.1039/C9NR10850E. - DOI - PubMed
-
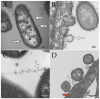
- Sheikh A., Zechmann B., Sayes C.M., Taube J.H., Greathouse K.L. A Preparation of Bacterial Outer Membrane with Osmium Tetroxide and Uranyl Acetate Co-Stain Enables Improved Structural Determination by Transmission Electron Microscopy. Microscopy. 2023:dfad027. doi: 10.1093/jmicro/dfad027. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

