Annual Report to the Nation on the Status of Cancer, part 2: Early assessment of the COVID-19 pandemic's impact on cancer diagnosis
- PMID: 37755665
- PMCID: PMC10841454
- DOI: 10.1002/cncr.35026
Annual Report to the Nation on the Status of Cancer, part 2: Early assessment of the COVID-19 pandemic's impact on cancer diagnosis
Abstract
Background: With access to cancer care services limited because of coronavirus disease 2019 control measures, cancer diagnosis and treatment have been delayed. The authors explored changes in the counts of US incident cases by cancer type, age, sex, race, and disease stage in 2020.
Methods: Data were extracted from selected US population-based cancer registries for diagnosis years 2015-2020 using first-submission data from the North American Association of Central Cancer Registries. After a quality assessment, the monthly numbers of newly diagnosed cancer cases were extracted for six cancer types: colorectal, female breast, lung, pancreas, prostate, and thyroid. The observed numbers of incident cancer cases in 2020 were compared with the estimated numbers by calculating observed-to-expected (O/E) ratios. The expected numbers of incident cases were extrapolated using Joinpoint trend models.
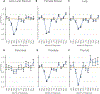
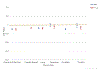
Results: The authors report an O/E ratio <1.0 for major screening-eligible cancer sites, indicating fewer newly diagnosed cases than expected in 2020. The O/E ratios were lowest in April 2020. For every cancer site except pancreas, Asians/Pacific Islanders had the lowest O/E ratio of any race group. O/E ratios were lower for cases diagnosed at localized stages than for cases diagnosed at advanced stages.
Conclusions: The current analysis provides strong evidence for declines in cancer diagnoses, relative to the expected numbers, between March and May of 2020. The declines correlate with reductions in pathology reports and are greater for cases diagnosed at in situ and localized stage, triggering concerns about potential poor cancer outcomes in the coming years, especially in Asians/Pacific Islanders.
Plain language summary: To help control the spread of coronavirus disease 2019 (COVID-19), health care organizations suspended nonessential medical procedures, including preventive cancer screening, during early 2020. Many individuals canceled or postponed cancer screening, potentially delaying cancer diagnosis. This study examines the impact of the COVID-19 pandemic on the number of newly diagnosed cancer cases in 2020 using first-submission, population-based cancer registry database. The monthly numbers of newly diagnosed cancer cases in 2020 were compared with the expected numbers based on past trends for six cancer sites. April 2020 had the sharpest decrease in cases compared with previous years, most likely because of the COVID-19 pandemic.
Keywords: cancer incidence; cancer stage; cancer surveillance; coronavirus disease 2019 (COVID-19); observed-to-expected; pathology reports.
© 2023 The Authors. Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
Conflict of Interest Statement
The other authors have no conflicts of interest to disclose.
Figures



References
-
- CMS Releases Recommendations on Adult Elective Surgeries, Non-Essential Medical, Surgical, and Dental Procedures During COVID-19 Response. CMS. Published online 2020. https://www.cms.gov/newsroom/press-releases/cms-releases-recommendations...
-
- Delayed Cancer Screenings. Epic Res. Published online May 4, 2020. https://epicresearch.org/articles/delays-in-preventive-cancer-screenings...
-
- Mast C, Munos del Rio A. Delayed Cancer Screenings- A Second Look. Epic Res. Published online July 17, 2020. https://epicresearch.org/articles/delayed-cancer-screenings-a-second-look/
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

