Prevalence and Overlap of Cardiac, Renal, and Metabolic Conditions in US Adults, 1999-2020
- PMID: 37755728
- PMCID: PMC10535010
- DOI: 10.1001/jamacardio.2023.3241
Prevalence and Overlap of Cardiac, Renal, and Metabolic Conditions in US Adults, 1999-2020
Abstract
Importance: Individually, cardiac, renal, and metabolic (CRM) conditions are common and leading causes of death, disability, and health care-associated costs. However, the frequency with which CRM conditions coexist has not been comprehensively characterized to date.
Objective: To examine the prevalence and overlap of CRM conditions among US adults currently and over time.
Design, setting, and participants: To establish prevalence of CRM conditions, nationally representative, serial cross-sectional data included in the January 2015 through March 2020 National Health and Nutrition Examination Survey (NHANES) were evaluated in this cohort study. To assess temporal trends in CRM overlap, NHANES data between 1999-2002 and 2015-2020 were compared. Data on 11 607 nonpregnant US adults (≥20 years) were included. Data analysis occurred between November 10, 2020, and November 23, 2022.
Main outcomes and measures: Proportion of participants with CRM conditions, overall and stratified by age, defined as cardiovascular disease (CVD), chronic kidney disease (CKD), type 2 diabetes (T2D), or all 3.
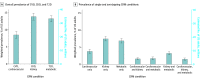
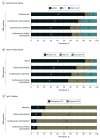
Results: From 2015 through March 2020, of 11 607 US adults included in the analysis (mean [SE] age, 48.5 [0.4] years; 51.0% women), 26.3% had at least 1 CRM condition, 8.0% had at least 2 CRM conditions, and 1.5% had 3 CRM conditions. Overall, CKD plus T2D was the most common CRM dyad (3.2%), followed by CVD plus T2D (1.7%) and CVD plus CKD (1.6%). Participants with higher CRM comorbidity burden were more likely to be older and male. Among participants aged 65 years or older, 33.6% had 1 CRM condition, 17.1% had 2 CRM conditions, and 5.0% had 3 CRM conditions. Within this subset, CKD plus T2D (7.3%) was most common, followed by CVD plus CKD (6.0%) and CVD plus T2D (3.8%). The CRM comorbidity burden was disproportionately high among participants reporting non-Hispanic Black race or ethnicity, unemployment, low socioeconomic status, and no high school degree. Among participants with 3 CRM conditions, nearly one-third (30.5%) did not report statin use, and only 4.8% and 3.0% used glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors, respectively. Between 1999 and 2020, the proportion of US adults with multiple CRM conditions increased significantly (from 5.3% to 8.0%; P < .001 for trend), as did the proportion having all 3 CRM conditions (0.7% to 1.5%; P < .001 for trend).
Conclusions and relevance: This cohort study found that CRM multimorbidity is increasingly common and undertreated among US adults, highlighting the importance of collaborative and comprehensive management strategies.
Conflict of interest statement
Figures
References
-
- Sarafidis P, Ferro CJ, Morales E, et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease: a consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol Dial Transplant. 2019;34(2):208-230. doi: 10.1093/ndt/gfy407 - DOI - PubMed
-
- Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration . Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2(8):634-647. doi: 10.1016/S2213-8587(14)70102-0 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

