The Protective Effect of Exogenous 17β-Estradiol against Experimentally Induced Oxidative Damage to Membrane Lipids Is Stronger in Male vs. Female Porcine Thyroids: Preliminary Results
- PMID: 37755756
- PMCID: PMC10535314
- DOI: 10.3390/toxics11090746
The Protective Effect of Exogenous 17β-Estradiol against Experimentally Induced Oxidative Damage to Membrane Lipids Is Stronger in Male vs. Female Porcine Thyroids: Preliminary Results
Abstract
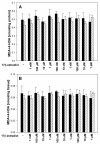
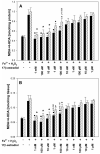
It is well-known that thyroid diseases are more prevalent in women than in men. The contribution of sex hormones may explain such disparity. The aim of this study was to check if there are any differences between sexes concerning the effects of 17β-estradiol on oxidative damage to membrane lipids (lipid peroxidation) in porcine thyroid homogenates under basal conditions and in the presence of Fenton reaction (Fe2+ + H2O2→Fe3+ + •OH + OH-) substrates. We observed that 17β-estradiol did not change the basal level of lipid peroxidation (measured spectrophotometrically as concentrations of malondialdehyde + 4-hydroxyalkenals) in thyroid homogenates, and no differences were found between sexes. The lipid peroxidation level in response to Fe2+ + H2O2 plus 17β-estradiol was lower in male thyroids. In turn, in male thyroids, 17β-estradiol reduced experimentally induced lipid peroxidation in as low of a concentration as 0.1 μM, whereas in female thyroids the lowest effective concentration of 17β-estradiol was 10 μM, i.e., 100 times higher than in males. In conclusion, the protective effects of exogenous 17β-estradiol against experimentally induced oxidative damage to membrane lipids is stronger in male than in female thyroids. Our observation suggests that female tissue is less sensitive to the protective effects of exogenous 17β-estradiol. This sexual dimorphism of oxidative processes in the thyroid may constitute one of the mechanisms of the different prevalence of thyroid diseases in women and in men.
Keywords: 17β-estradiol; Fenton reaction; oxidative stress; sexual dimorphism; thyroid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Fenton Reaction-Induced Oxidative Damage to Membrane Lipids and Protective Effects of 17β-Estradiol in Porcine Ovary and Thyroid Homogenates.Int J Environ Res Public Health. 2020 Sep 18;17(18):6841. doi: 10.3390/ijerph17186841. Int J Environ Res Public Health. 2020. PMID: 32962175 Free PMC article.
-
17β-estradiol prevents experimentally-induced oxidative damage to membrane lipids and nuclear DNA in porcine ovary.Syst Biol Reprod Med. 2016;62(1):17-21. doi: 10.3109/19396368.2015.1101510. Epub 2015 Dec 17. Syst Biol Reprod Med. 2016. PMID: 26677908
-
Oxidative damage to membrane lipids in the thyroid - no differences between sexes.Drug Chem Toxicol. 2021 Nov;44(6):655-660. doi: 10.1080/01480545.2019.1643878. Epub 2019 Aug 2. Drug Chem Toxicol. 2021. PMID: 31373249
-
Potassium iodide, but not potassium iodate, as a potential protective agent against oxidative damage to membrane lipids in porcine thyroid.Thyroid Res. 2013 Aug 30;6(1):10. doi: 10.1186/1756-6614-6-10. Thyroid Res. 2013. PMID: 24004681 Free PMC article.
-
Sexual dimorphism and thyroid dysfunction: a matter of oxidative stress?J Endocrinol. 2014 Apr 22;221(2):R31-40. doi: 10.1530/JOE-13-0588. Print 2014 May. J Endocrinol. 2014. PMID: 24578296 Review.
Cited by
-
17β-Estradiol Stimulates Oxidative Stress Components and Thyroid Specific Genes in Porcine Thyroid Follicular Cells: Potential Differences Between Sexes.Cells. 2024 Oct 25;13(21):1769. doi: 10.3390/cells13211769. Cells. 2024. PMID: 39513876 Free PMC article.
References
-
- Clocchiatti A., Cora E., Zhang Y., Dotto G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer. 2016;16:330–339. - PubMed
-
- James B.C., Mitchell J.M., Jeon H.D., Vasilottos N., Grogan R.H., Aschebrook-Kilfoy B. An update in international trends in incidence rates of thyroid cancer, 1973–2007. Cancer Causes Control. 2018;29:465–473. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

