Study on Dihydromyricetin Improving Aflatoxin Induced Liver Injury Based on Network Pharmacology and Molecular Docking
- PMID: 37755770
- PMCID: PMC10535947
- DOI: 10.3390/toxics11090760
Study on Dihydromyricetin Improving Aflatoxin Induced Liver Injury Based on Network Pharmacology and Molecular Docking
Abstract
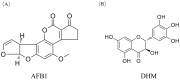
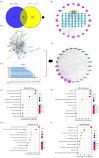
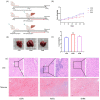
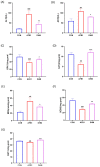
Aflatoxin B1 (AFB1) is a toxic food/feed contaminant and the liver is its main target organ, thus it poses a great danger to organisms. Dihydromyricetin (DHM), a natural flavonoid compound, can be used as a food additive with high safety and has been shown to have strong hepatoprotective effects. In this experiment, PPI network and KEGG pathway analysis were constructed by network pharmacological analysis technique using software and platforms such as Swiss, String, and David and Cytoscape. We screened AFB1 and DHM cross-targets and pathways of action, followed by molecular docking based on the strength of binding affinity of genes to DHM. In addition, we exposed AFB1 (200 μg/kg) to mice to establish a liver injury model. Histological observation, biochemical assay, oxidative stress indicator assay, TUNEL staining and Western blot were used to evaluate the liver injury. Network pharmacological results were screened to obtain 25 cross-targets of action and 20 pathways of action. It was found that DHM may exert anti-hepatic injury effects by inhibiting the overexpression of Caspase-3 protein and increasing the expression of Bcl-2 protein. DHM (200 mg/kg) was found to reduce AFB1-induced liver indices such as alanine aminotransferase (ALT) and aspartate acyltransferase (AST), and attenuate hepatic histopathological damage through animal models. Importantly, DHM inhibited malondialdehyde (MDA) formation in liver tissue and attenuated AFB1-induced oxidative stress injury by increasing glutathione-S-transferase (GST) glutathione (GPX) catalase (CAT) and superoxide dismutase (SOD). Meanwhile, DHM also restored the expression of anti-apoptotic protein Bcl-2 and antioxidant proteins, Nrf2, Keap1 and its downstream HO-1, and down-regulated the expression of pro-apoptotic proteins Bax and Caspase-3 in AFB1-induced liver tissues. The results confirmed that liver injury caused by AFB1 exposure could be alleviated by DHM, providing valuable guidance for in-depth study of DHM in the treatment of liver-related diseases, and laying the foundation for in-depth development and utilization of DHM.
Keywords: aflatoxin; dihydromyricetin; liver injury; network pharmacology; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Taraxasterol alleviates aflatoxin B1-induced liver damage in broiler chickens via regulation of oxidative stress, apoptosis and autophagy.Ecotoxicol Environ Saf. 2023 Feb;251:114546. doi: 10.1016/j.ecoenv.2023.114546. Epub 2023 Jan 14. Ecotoxicol Environ Saf. 2023. PMID: 36646010
-
Marine algal polysaccharides alleviate aflatoxin B1-induced bursa of Fabricius injury by regulating redox and apoptotic signaling pathway in broilers.Poult Sci. 2021 Feb;100(2):844-857. doi: 10.1016/j.psj.2020.10.050. Epub 2020 Nov 4. Poult Sci. 2021. PMID: 33518138 Free PMC article.
-
Salvia miltiorrhiza polysaccharide mitigates AFB1-induced liver injury in rabbits.Ecotoxicol Environ Saf. 2024 May;276:116344. doi: 10.1016/j.ecoenv.2024.116344. Epub 2024 Apr 17. Ecotoxicol Environ Saf. 2024. PMID: 38636259
-
Multiple molecular and cellular mechanisms of the antitumour effect of dihydromyricetin (Review).Biomed Rep. 2024 Mar 26;20(5):82. doi: 10.3892/br.2024.1769. eCollection 2024 May. Biomed Rep. 2024. PMID: 38628627 Free PMC article. Review.
-
Dihydromyricetin: an emerging compound with comprehensive effects on multiple systems.Front Pharmacol. 2025 Jan 3;15:1488003. doi: 10.3389/fphar.2024.1488003. eCollection 2024. Front Pharmacol. 2025. PMID: 39830336 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

