Unveiling the Clinical Benefits of High-Volume Hemodiafiltration: Optimizing the Removal of Medium-Weight Uremic Toxins and Beyond
- PMID: 37755957
- PMCID: PMC10535648
- DOI: 10.3390/toxins15090531
Unveiling the Clinical Benefits of High-Volume Hemodiafiltration: Optimizing the Removal of Medium-Weight Uremic Toxins and Beyond
Abstract
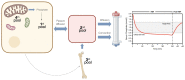
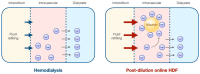
Dialysis treatment has improved the survival of patients with kidney failure. However, the hospitalization and mortality rates remain alarmingly high, primarily due to incomplete uremic toxin elimination. High-volume hemodiafiltration (HDF) has emerged as a promising approach that significantly improves patient outcomes by effectively eliminating medium and large uremic toxins, which explains its increasing adoption, particularly in Europe and Japan. Interest in this therapy has grown following the findings of the recently published CONVINCE study, as well as the need to understand the mechanisms behind the benefits. This comprehensive review aims to enhance the scientific understanding by explaining the underlying physiological mechanisms that contribute to the positive effects of HDF in terms of short-term benefits, like hemodynamic tolerance and cardiovascular disease. Additionally, it explores the rationale behind the medium-term clinical benefits, including phosphorus removal, the modulation of inflammation and oxidative stress, anemia management, immune response modulation, nutritional effects, the mitigation of bone disorders, neuropathy relief, and amyloidosis reduction. This review also analyzes the impact of HDF on patient-reported outcomes and mortality. Considering the importance of applying personalized uremic toxin removal strategies tailored to the unique needs of each patient, high-volume HDF appears to be the most effective treatment to date for patients with renal failure. This justifies the need to prioritize its application in clinical practice, initially focusing on the groups with the greatest potential benefits and subsequently extending its use to a larger number of patients.
Keywords: cardiovascular disease; dialysis; hemodiafiltration; hemodynamic tolerance; inflammation; kidney failure; mortality; oxidative stress; patient-reported outcomes; uremic toxins.
Conflict of interest statement
C.P.-R., A.J., E.L. and S.M. have received honoraria (paid to employer) from Fresenius Medical Care for their participation in the Advisory Board and educational activities. R.P.-F. is employed by Arbor Research Collaborative for Health, who runs the DOPPS studies. Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. Funding is provided to Arbor Research Collaborative for Health and not to Pecoits-Filho directly. P.H. is employed part-time by Fresenius Medica Care Chile. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

