Lactobacillus fermentum Alleviates the Colorectal Inflammation Induced by Low-Dose Sub-Chronic Microcystin-LR Exposure
- PMID: 37756005
- PMCID: PMC10536654
- DOI: 10.3390/toxins15090579
Lactobacillus fermentum Alleviates the Colorectal Inflammation Induced by Low-Dose Sub-Chronic Microcystin-LR Exposure
Abstract
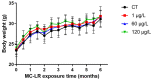
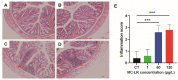
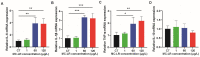
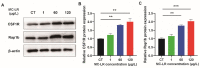
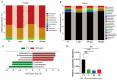
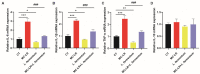
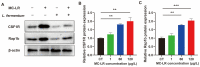
Microcystin-LR (MC-LR) contamination is a worldwide environmental problem that poses a grave threat to the water ecosystem and public health. Exposure to MC-LR has been associated with the development of intestinal injury, but there are no effective treatments for MC-LR-induced intestinal disease. Probiotics are "live microorganisms that are beneficial to the health of the host when administered in sufficient quantities". It has been demonstrated that probiotics can prevent or treat a variety of human diseases; however, their ability to mitigate MC-LR-induced intestinal harm has not yet been investigated. The objective of this study was to determine whether probiotics can mitigate MC-LR-induced intestinal toxicity and its underlying mechanisms. We first evaluated the pathological changes in colorectal tissues using an animal model with sub-chronic exposure to low-dose MC-LR, HE staining to assess colorectal histopathologic changes, qPCR to detect the expression levels of inflammatory factors in colorectal tissues, and WB to detect the alterations on CSF1R signaling pathway proteins in colorectal tissues. Microbial sequencing analysis and screening of fecal microorganisms differential to MC-LR treatment in mice. To investigate the role of microorganisms in MC-LR-induced colorectal injury, an in vitro model of MC-LR co-treatment with microorganisms was developed. Our findings demonstrated that MC-LR treatment induced an inflammatory response in mouse colorectal tissues, promoted the expression of inflammatory factors, activated the CSF1R signaling pathway, and significantly decreased the abundance of Lactobacillus. In a model of co-treatment with MC-LR and Lactobacillus fermentum (L. fermentum), it was discovered that L. fermentum substantially reduced the incidence of the colorectal inflammatory response induced by MC-LR and inhibited the protein expression of the CSF1R signaling pathway. This is the first study to suggest that L. fermentum inhibits the CSF1R signaling pathway to reduce the incidence of MC-LR-induced colorectal inflammation. This research may provide an excellent experimental foundation for the development of strategies for the prevention and treatment of intestinal diseases in MC-LR.
Keywords: Lactobacillus fermentum; colorectal inflammation; intestinal diseases; microcystin-LR; probiotics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Long-term environmental levels of microcystin-LR exposure induces colorectal chronic inflammation, fibrosis and barrier disruption via CSF1R/Rap1b signaling pathway.J Hazard Mater. 2022 Oct 15;440:129793. doi: 10.1016/j.jhazmat.2022.129793. Epub 2022 Aug 18. J Hazard Mater. 2022. PMID: 36029734
-
Subchronic Microcystin-LR Aggravates Colorectal Inflammatory Response and Barrier Disruption via Raf/ERK Signaling Pathway in Obese Mice.Toxins (Basel). 2023 Apr 1;15(4):262. doi: 10.3390/toxins15040262. Toxins (Basel). 2023. PMID: 37104200 Free PMC article.
-
Exposure to microcystin-LR promotes the progression of colitis-associated colorectal cancer by inducing barrier disruption and gut microbiota dysbiosis.Ecotoxicol Environ Saf. 2024 Sep 1;282:116750. doi: 10.1016/j.ecoenv.2024.116750. Epub 2024 Jul 24. Ecotoxicol Environ Saf. 2024. PMID: 39053045
-
Effect of chronic low-dose microcystin-LR exposure on jejunum apoptosis via RAF/ERK signaling pathway in mouse.J Toxicol Environ Health A. 2025 Apr 3;88(7):291-300. doi: 10.1080/15287394.2024.2435631. Epub 2024 Dec 12. J Toxicol Environ Health A. 2025. PMID: 39668503
-
Lactobacillus fermentum and Lactobacillus plantarum increased gut microbiota diversity and functionality, and mitigated Enterobacteriaceae, in a mouse model.Benef Microbes. 2019 Apr 19;10(4):413-424. doi: 10.3920/BM2018.0074. Epub 2019 Apr 8. Benef Microbes. 2019. PMID: 30957532
Cited by
-
Serum Microcystin-LR Levels Linked with Risk of Inflammatory Bowel Disease: A Matched Case-Control Study in China.Environ Health (Wash). 2024 May 3;2(7):453-464. doi: 10.1021/envhealth.3c00212. eCollection 2024 Jul 19. Environ Health (Wash). 2024. PMID: 39473886 Free PMC article.
-
Astaxanthin Alleviates Hepatic Lipid Metabolic Dysregulation Induced by Microcystin-LR.Toxins (Basel). 2024 Sep 18;16(9):401. doi: 10.3390/toxins16090401. Toxins (Basel). 2024. PMID: 39330859 Free PMC article.
-
Effects of different doses of microcystin-LR exposure on gut development and the microbiota of Xenopus laevis tadpoles.BMC Microbiol. 2025 Jul 2;25(1):395. doi: 10.1186/s12866-025-04085-2. BMC Microbiol. 2025. PMID: 40604410 Free PMC article.
References
-
- Feng S., Deng S., Tang Y., Liu Y., Yang Y., Xu S., Tang P., Lu Y., Duan Y., Wei J., et al. Microcystin-LR Combined with Cadmium Exposures and the Risk of Chronic Kidney Disease: A Case-Control Study in Central China. Environ. Sci. Technol. 2022;56:15818–15827. doi: 10.1021/acs.est.2c02287. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

