Comparing Traditional and Toxin-Oriented Approaches towards Antivenom Production against Bitis arietans Snake Venom
- PMID: 37756010
- PMCID: PMC10537286
- DOI: 10.3390/toxins15090584
Comparing Traditional and Toxin-Oriented Approaches towards Antivenom Production against Bitis arietans Snake Venom
Abstract
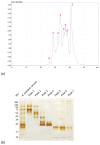
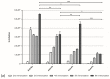
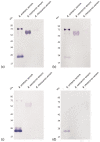
Accidents with snakes are responsible for about 32,000 deaths annually in sub-Saharan Africa, caused mostly by snakes from the genus Bitis, in particular Bitis arietans. B. arietans venom is composed of a complex mixture of toxins, mainly metalloproteases, serine proteases, phospholipases, lectins, and disintegrins. In this work, we compared two approaches to anti-B. arietans antivenom production: immunization with crude snake venom ("traditional approach") and immunization with selected key toxins isolated from the snake venom ("toxin oriented" approach). Fractions from B. arietans venom were isolated by size exclusion chromatography. Crude venom and samples containing serine proteases or metalloproteases were selected for the immunization of BALB/c mice. Anti-B. arietans and anti-serine proteases plasmas showed a similar recognition profile and higher titers and affinity than the anti-metalloproteases plasma. Cross-recognition of other Bitis venoms was observed, but with low intensity. Although the plasma of all experimental groups inhibited the enzymatic activity of B. arietans venom in vitro, in vivo protection was not achieved. Our results have shown limitations in both approaches considered. Based on this, we proposed a model of polyclonal, species-specific, monovalent antivenoms that could be used as a base to produce customizable polyvalent sera for use in sub-Saharan Africa.
Keywords: Bitis; Bitis arietans; antibody; antivenom; snake venom; sub-Saharan Africa.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kasturiratne A., Wickremasinghe A.R., Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R., Savioli L., Lalloo D.G., de Silva H.J. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med. 2008;5:218. doi: 10.1371/journal.pmed.0050218. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

