Microbiome Responses to Fecal Microbiota Transplantation in Cats with Chronic Digestive Issues
- PMID: 37756083
- PMCID: PMC10537086
- DOI: 10.3390/vetsci10090561
Microbiome Responses to Fecal Microbiota Transplantation in Cats with Chronic Digestive Issues
Abstract
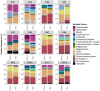
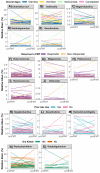
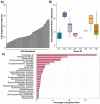
There is growing interest in the application of fecal microbiota transplants (FMTs) in small animal medicine, but there are few published studies that have tested their effects in the domestic cat (Felis catus). Here we use 16S rRNA gene sequencing to examine fecal microbiome changes in 46 domestic cats with chronic digestive issues that received FMTs using lyophilized stool that was delivered in oral capsules. Fecal samples were collected from FMT recipients before and two weeks after the end of the full course of 50 capsules, as well as from their stool donors (N = 10), and other healthy cats (N = 113). The fecal microbiomes of FMT recipients varied with host clinical signs and dry kibble consumption, and shifts in the relative abundances of Clostridium, Collinsella, Megamonas, Desulfovibrio and Escherichia were observed after FMT. Overall, donors shared 13% of their bacterial amplicon sequence variants (ASVs) with FMT recipients and the most commonly shared ASVs were classified as Prevotella 9, Peptoclostridium, Bacteroides, and Collinsella. Lastly, the fecal microbiomes of cats with diarrhea became more similar to the microbiomes of age-matched and diet-matched healthy cats compared to cats with constipation. Overall, our results suggest that microbiome responses to FMT may be modulated by the FMT recipient's initial presenting clinical signs, diet, and their donor's microbiome.
Keywords: FMT; antibiotics; diarrhea; domestic cats; dysbiosis; fecal microbiome; fecal transplant; gut microbiome; vomiting.
Conflict of interest statement
C.A.R., H.H.G, J.K.G., G.J., D.D.K., A.M. and Z.E. are employees of AnimalBiome, a company based in Oakland, CA and have stock options. J.A.E. is an advisor for AnimalBiome and has stock options.
Figures



References
-
- Ganz H.H., Jospin G., Rojas C.A., Martin A.L., Dahlhausen K., Kingsbury D.D., Osborne C.X., Entrolezo Z., Redner S., Ramirez B., et al. The Kitty Microbiome Project: Defining the Healthy Fecal “Core Microbiome” in Pet Domestic Cats. Vet. Sci. 2022;9:635. doi: 10.3390/vetsci9110635. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

