Secreted folate receptor γ drives fibrogenesis in metabolic dysfunction-associated steatohepatitis by amplifying TGFβ signaling in hepatic stellate cells
- PMID: 37756380
- PMCID: PMC11816833
- DOI: 10.1126/scitranslmed.ade2966
Secreted folate receptor γ drives fibrogenesis in metabolic dysfunction-associated steatohepatitis by amplifying TGFβ signaling in hepatic stellate cells
Abstract
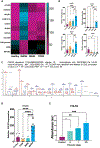
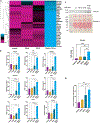
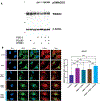
Hepatic fibrosis is the primary determinant of mortality in patients with metabolic dysfunction-associated steatohepatitis (MASH). Transforming growth factor-β (TGFβ), a master profibrogenic cytokine, is a promising therapeutic target that has not yet been translated into an effective therapy in part because of liabilities associated with systemic TGFβ antagonism. We have identified that soluble folate receptor γ (FOLR3), which is expressed in humans but not in rodents, is a secreted protein that is elevated in the livers of patients with MASH but not in those with metabolic dysfunction-associated steatotic liver disease, those with type II diabetes, or healthy individuals. Global proteomics showed that FOLR3 was the most highly significant MASH-specific protein and was positively correlated with increasing fibrosis stage, consistent with stimulation of activated hepatic stellate cells (HSCs), which are the key fibrogenic cells in the liver. Exposure of HSCs to exogenous FOLR3 led to elevated extracellular matrix (ECM) protein production, an effect synergistically potentiated by TGFβ1. We found that FOLR3 interacts with the serine protease HTRA1, a known regulator of TGFBR, and activates TGFβ signaling. Administration of human FOLR3 to mice induced severe bridging fibrosis and an ECM pattern resembling human MASH. Our study thus uncovers a role of FOLR3 in enhancing fibrosis.
Conflict of interest statement
Figures
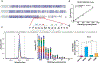


References
-
- Hossain P, Kawar B, El Nahas M, Obesity and diabetes in the developing world — A growing challenge. N. Engl. J. Med. 356, 213–215 (2007). - PubMed
-
- Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E, Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 15, 11–20 (2018). - PubMed
-
- Younossi ZM, Henry L, The impact of obesity and type 2 diabetes on chronic liver disease. Am. J. Gastroenterol. 114, 1714–1715 (2019). - PubMed
-
- Younossi ZM, Marchesini G, Pinto-Cortez H, Petta S, Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Implications for liver transplantation. Transplantation 103, 22–27 (2019). - PubMed
-
- Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, Kechagias S, Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 67, 1265–1273 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

