Understanding yellow fever-associated myocardial injury: an autopsy study
- PMID: 37757571
- PMCID: PMC10550587
- DOI: 10.1016/j.ebiom.2023.104810
Understanding yellow fever-associated myocardial injury: an autopsy study
Abstract
Background: Yellow fever (YF) is a viral hemorrhagic fever, endemic in parts of South America and Africa. There is scarce evidence about the pathogenesis of the myocardial injury. The objective of this study is to evaluate the cardiac pathology in fatal cases of YF.
Methods: This retrospective autopsy study included cases from the São Paulo (Brazil) epidemic of 2017-2019. We reviewed medical records and performed cardiac tissue histopathological evaluation, electron microscopy, immunohistochemical assays, RT-qPCR for YF virus (YFV)-RNA, and proteomics analysis on inflammatory and endothelial biomarkers.
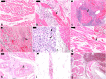
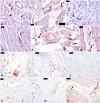
Findings: Seventy-three confirmed YF cases with a median age of 48 (34-60) years were included. We observed myocardial fibrosis in 68 (93.2%) patients; cardiomyocyte hypertrophy in 68 (93.2%); endothelial alterations in 67 (91.8%); fiber necrosis in 50 (68.5%); viral myocarditis in 9 (12.3%); and secondary myocarditis in 5 (6.8%). Four out of five patients with 17DD vaccine-associated viscerotropic disease presented with myocarditis. The cardiac conduction system showed edema, hemorrhages and endothelial fibrinoid necrosis. Immunohistochemistry detected CD68-positive inflammatory interstitial cells and YFV antigens in endothelial and inflammatory cells. YFV-RNA was detected positive in 95.7% of the cardiac samples. The proteomics analysis demonstrated that YF patients had higher levels of multiple inflammatory and endothelial biomarkers in comparison to cardiovascular controls, and higher levels of interferon gamma-induced protein 10 (IP-10) in comparison to sepsis (p = 0.01) and cardiovascular controls (p < 0.001) in Dunn test.
Interpretation: Myocardial injury is frequent in severe YF, due to multifactorial mechanisms, including direct YFV-mediated damage, endothelial cell injury, and inflammatory response, with a possible prominent role for IP-10.
Funding: This study was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo, Bill and Melinda Gates Foundation, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior.
Keywords: 17DD; Autopsy; Chemokines; Cytokines; Heart conduction system; IP-10; Myocarditis; Vascular endothelium; YEL-AVD; Yellow fever.
Copyright © 2023 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflict of interests.
Figures



References
-
- Tuells J., Henao-Martínez A.F., Franco-Paredes C. Yellow fever: a perennial threat. Arch Med Res. 2022;53:649–657. - PubMed
-
- Eliminate Yellow fever Epidemics (EYE): a global strategy, 2017–2026. Wkly Epidemiol Rec. 2017;92:193–204. - PubMed
-
- Monath T.P., Vasconcelos P.F.C. Yellow fever. J Clin Virol. 2015;64:160–173. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

