Considerations for Colorectal Neoplasia Detection in Inflammatory Bowel Disease Clinical Trials
- PMID: 37757769
- PMCID: PMC10836758
- DOI: 10.1159/000533395
Considerations for Colorectal Neoplasia Detection in Inflammatory Bowel Disease Clinical Trials
Abstract
Background: High-quality colonoscopic surveillance can lead to earlier and increased detection of colorectal neoplasia in patients with inflammatory bowel disease (IBD). In IBD clinical trials, endoscopy is used to assess mucosal disease activity before and after treatment but also provides an opportunity to surveil for colorectal neoplasia during follow-up.
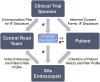
Summary: Best practices for colorectal cancer identification in IBD clinical trials require engagement and collaboration between the clinical trial sponsor, site endoscopist and/or principal investigator, and central read team. Each team member has unique responsibilities for maximizing dysplasia detection in IBD trials.
Key messages: Sponsors should work in accordance with scientific guidelines to standardize imaging procedures, design the protocol to ensure the trial population is safeguarded, and oversee trial conduct. The site endoscopist should remain updated on best practices to tailor sponsor protocol-required procedures to patient needs, examine the mucosa for disease activity and potential dysplasia during all procedures, and provide optimal procedure videos for central read analysis. Central readers may detect dysplasia or colorectal cancer and a framework to report these findings to trial sponsors is essential. Synergistic relationships between all team members in IBD clinical trials provide an important opportunity for extended endoscopic evaluation and colorectal neoplasia identification.
Keywords: Clinical trial; Colorectal cancer; Colorectal neoplasms; Endoscopy; Inflammatory bowel disease.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
M.M.Y. was an employee of Bristol Myers Squibb at the time of manuscript initiation. H.A.A., S.A., and A.S. are employees and shareholders of Bristol Myers Squibb. J.B.C. was an employee of Bristol Myers Squibb at the time of manuscript submission. K.U. was an employee of Bristol Myers Squibb at the time of manuscript initiation and currently serves as a paid consultant. F.A.F. has received consulting fees from Arena, BMS, Braintree Labs, GI Reviewers, GSK, IBD Educational Group, Innovation Pharmaceuticals, Iterative Scopes, Janssen, Pfizer, and Sebela. He serves on a data safety monitoring board for Bacainn Therapeutics, Lilly, and Theravance. C.M. has received consulting fees from AbbVie, Amgen, AVIR Pharma Inc, Ferring, Fresenius Kabi, Janssen, McKesson, Mylan, Takeda, Pfizer, Roche, and Alimentiv (formerly Robarts Clinical Trials Inc.); speaker’s fees from AbbVie, AVIR Pharma Inc, Janssen, Takeda, and Pfizer; and research support from Pfizer.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

