Rapid Peroxide Removal Limits the Radiosensitization of Diffuse Intrinsic Pontine Glioma (DIPG) Cells by Pharmacologic Ascorbate
- PMID: 37758035
- PMCID: PMC10759934
- DOI: 10.1667/RADE-23-00006.1
Rapid Peroxide Removal Limits the Radiosensitization of Diffuse Intrinsic Pontine Glioma (DIPG) Cells by Pharmacologic Ascorbate
Abstract
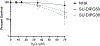
Diffuse intrinsic pontine gliomas (DIPG) are an aggressive type of pediatric brain tumor with a very high mortality rate. Surgery has a limited role given the tumor's location. Palliative radiation therapy alleviates symptoms and prolongs survival, but median survival remains less than 1 year. There is no clear role for chemotherapy in DIPGs as trials adding chemotherapy to palliative radiation therapy have failed to improve survival compared to radiation alone. Thus, there is a critical need to identify tissue-specific radiosensitizers to improve clinical outcomes for patients with DIPGs. Pharmacologic (high dose) ascorbate (P-AscH-) is a promising anticancer therapy that sensitizes human tumors, including adult high-grade gliomas, to radiation by acting selectively as a generator of hydrogen peroxide (H2O2) in cancer cells. In this study we demonstrate that in contrast to adult glioma models, P-AscH- does not radiosensitize DIPG. DIPG cells were sensitive to bolus of H2O2 but have faster H2O2 removal rates than GBM models which are radiosensitized by P-AscH-. These data support the hypothesis that P-AscH- does not enhance DIPG radiosensitivity, likely due to a robust capacity to detoxify and remove hydroperoxides.
©2023 by Radiation Research Society. All rights of reproduction in any form reserved.
Figures

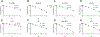
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

