Microphysiological Modeling of Gingival Tissues and Host-Material Interactions Using Gingiva-on-Chip
- PMID: 37758297
- PMCID: PMC11468103
- DOI: 10.1002/adhm.202301472
Microphysiological Modeling of Gingival Tissues and Host-Material Interactions Using Gingiva-on-Chip
Abstract
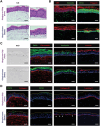
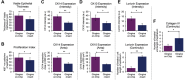
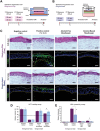
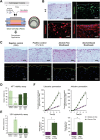
Gingiva plays a crucial barrier role at the interface of teeth, tooth-supporting structures, microbiome, and external agents. To mimic this complex microenvironment, an in vitro microphysiological platform and biofabricated full-thickness gingival equivalents (gingiva-on-chip) within a vertically stacked microfluidic device is developed. This design allowed long-term and air-liquid interface culture, and host-material interactions under flow conditions. Compared to static cultures, dynamic cultures on-chip enabled the biofabrication of gingival equivalents with stable mucosal matrix, improved epithelial morphogenesis, and barrier features. Additionally, a diseased state with disrupted barrier function representative of gingival/oral mucosal ulcers is modeled. The apical flow feature is utilized to emulate the mechanical action of mouth rinse and integrate the assessment of host-material interactions and transmucosal permeation of oral-care formulations in both healthy and diseased states. Although the gingiva-on-chip cultures have thicker and more mature epithelium, the flow of oral-care formulations induced increased tissue disruption and cytotoxic features compared to static conditions. The realistic emulation of mouth rinsing action facilitated a more physiological assessment of mucosal irritation potential. Overall, this microphysiological system enables biofabrication of human gingiva equivalents in intact and ulcerated states, providing a miniaturized and integrated platform for downstream host-material and host-microbiome applications in gingival and oral mucosa research.
Keywords: biocompatibility; drug permeation; gingiva; microfluidics; organ-on-a-chip; periodontal disease.
© 2023 The Authors. Advanced Healthcare Materials published by Wiley-VCH GmbH.
Conflict of interest statement
The authors would also like to disclose that two of the authors, M.A. and G.S., are co‐founders of Revivo Biosystems Pte Ltd., and the microfluidic device used in this study is based on a patent application (WO2018030958A1) licensed to the company.
Figures


References
-
- Klausner M., Ayehunie S., Breyfogle B. A., Wertz P. W., Bacca L., Kubilus J., Toxicol. In Vitro 2007, 21, 938. - PubMed
-
- Viñuela‐Prieto J. M., Sánchez‐Quevedo M. C., Alfonso‐Rodríguez C. A., Oliveira A. C., Scionti G., Martín‐Piedra M. A., Moreu G., Campos A., Alaminos M., Garzón I., J. Periodontal Res. 2015, 50, 658. - PubMed
-
- Presland R. B., Dale B. A., Crit. Rev. Oral Biol. Med. 2000, 11, 383. - PubMed
-
- Ibrahim M. S., El‐Wassefy N. A., Farahat D. S., Biomaterials for Oral and Dental Tissue Engineering, Woodhead Publishing, Cambridge: 2017.
Publication types
MeSH terms
Grants and funding
- A-0002944-00-00/National University of Singapore
- A-0002944-01-00/National University of Singapore
- ING-000158 BIO/Singapore-MIT Alliance for Research and Technology Centre
- ACCL-19-GAP001-R20H/Agency for Science, Technology and Research
- NUHSRO/2021/107/RO5+6/Seed-Sep/10/National University Health System
LinkOut - more resources
Full Text Sources

