AlphaFold-Multimer predicts cross-kingdom interactions at the plant-pathogen interface
- PMID: 37758696
- PMCID: PMC10533508
- DOI: 10.1038/s41467-023-41721-9
AlphaFold-Multimer predicts cross-kingdom interactions at the plant-pathogen interface
Abstract
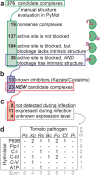
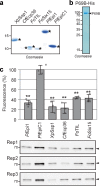
Adapted plant pathogens from various microbial kingdoms produce hundreds of unrelated small secreted proteins (SSPs) with elusive roles. Here, we used AlphaFold-Multimer (AFM) to screen 1879 SSPs of seven tomato pathogens for interacting with six defence-related hydrolases of tomato. This screen of 11,274 protein pairs identified 15 non-annotated SSPs that are predicted to obstruct the active site of chitinases and proteases with an intrinsic fold. Four SSPs were experimentally verified to be inhibitors of pathogenesis-related subtilase P69B, including extracellular protein-36 (Ecp36) and secreted-into-xylem-15 (Six15) of the fungal pathogens Cladosporium fulvum and Fusarium oxysporum, respectively. Together with a P69B inhibitor from the bacterial pathogen Xanthomonas perforans and Kazal-like inhibitors of the oomycete pathogen Phytophthora infestans, P69B emerges as an effector hub targeted by different microbial kingdoms, consistent with a diversification of P69B orthologs and paralogs. This study demonstrates the power of artificial intelligence to predict cross-kingdom interactions at the plant-pathogen interface.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

