Omicron variant neutralizing antibodies following BNT162b2 BA.4/5 versus mRNA-1273 BA.1 bivalent vaccination in patients with end-stage kidney disease
- PMID: 37758707
- PMCID: PMC10533557
- DOI: 10.1038/s41467-023-41678-9
Omicron variant neutralizing antibodies following BNT162b2 BA.4/5 versus mRNA-1273 BA.1 bivalent vaccination in patients with end-stage kidney disease
Abstract
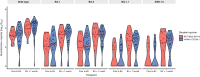
Neutralization of Omicron subvariants by different bivalent vaccines has not been well evaluated. This study characterizes neutralization against Omicron subvariants in 98 individuals on dialysis or with a kidney transplant receiving the BNT162b2 (BA.4/BA.5) or mRNA-1273 (BA.1) bivalent COVID-19 vaccine. Neutralization against Omicron BA.1, BA.5, BQ.1.1, and XBB.1.5 increased by 8-fold one month following bivalent vaccination. In comparison to wild-type (D614G), neutralizing antibodies against Omicron-specific variants were 7.3-fold lower against BA.1, 8.3-fold lower against BA.5, 45.8-fold lower against BQ.1.1, and 48.2-fold lower against XBB.1.5. Viral neutralization was not significantly different by bivalent vaccine type for wild-type (D614G) (P = 0.48), BA.1 (P = 0.21), BA.5 (P = 0.07), BQ.1.1 (P = 0.10), nor XBB.1.5 (P = 0.10). Hybrid immunity conferred higher neutralizing antibodies against all Omicron subvariants. This study provides evidence that BNT162b2 (BA.4/BA.5) and mRNA-1273 (BA.1) induce similar neutralization against Omicron subvariants, even when antigenically divergent from the circulating variant.
© 2023. The Author(s).
Conflict of interest statement
A.L. reports being a scientific advisor to, or member of, AstraZeneca, Bayer, Boehringer-Ingelheim, Canadian Journal of Kidney Health and Disease, Canadian Institutes of Health Research, Certa, Chinook Therapeutics, Johnson and Johnson, Kidney Foundation of Canada, National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Otsuka, Reata, Retrophin, and The George Institute; receiving research funding from AstraZeneca, Boehringer-Ingelheim, Canadian Institute of Health Research, Janssen, Johnson and Johnson, Kidney Foundation of Canada, Merck, NIDDK, NIH, Ortho Biotech, Otsuka, and Oxford Clinical Trials; and having consultancy agreements with Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Johnson and Johnson/Jansen, Reata, and Retrophin (unrelated to the submitted work). D.Y. reports being a scientific co-founder and consultant for Fibrocor Therapeutics; receiving speaking honoraria and/or consultancy fees from AstraZeneca, GlaxoSmithKline, and Vivace Therapeutics (unrelated to the submitted work). J.P. reported receiving speaking honoraria and consultancy fees from Baxter Healthcare; grants from Agency for Healthcare Research and Quality grant support; speaking honoraria from Fresenius Medical Care, AstraZeneca, Davita Healthcare, and US Renal Care; and consultancy fees from LiberDi Dialysis (unrelated to the submitted work). J.L. has received payment for expert testimony upon request of hospitals of the Ontario Hospital Association, Ministry of Attorney General of Ontario, and Seneca College (unrelated to the submitted work). A.C.G. has received research funds from a research contract with Providence Therapeutics Holdings, Inc for other projects, participated in the COVID-19 Immunity Task Force Immune Science and Testing working party, chaired the CIHR Institute of Genetics Advisory Board, and chairs the SAB of the National Research Council of Canada Human Health Therapeutics Board. M.O. and M.H are contracted Medical Leads at the Ontario Renal Network, Ontario Health. Matthew Oliver is owner of Oliver Medical Management Inc., which licenses Dialysis Management Analysis and Reporting System software. He has received honoraria for speaking from Baxter Healthcare (unrelated to the submitted work). M.H. reports receiving grants from Pfizer for a study in focal segmental glomerulosclerosis, Ionis, Calliditas and Chinook for studies in Immunoglobulin A nephropathy, and Roche for a preeclampsia study (unrelated to the submitted work). No other competing interests were declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

