Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients
- PMID: 37758751
- PMCID: PMC10533894
- DOI: 10.1038/s41541-023-00742-7
Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients
Abstract
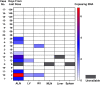
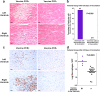
At the start of the COVID-19 pandemic, the BNT162b2 (BioNTech-Pfizer) and mRNA-1273 (Moderna) mRNA vaccines were expediently designed and mass produced. Both vaccines produce the full-length SARS-CoV-2 spike protein for gain of immunity and have greatly reduced mortality and morbidity from SARS-CoV-2 infection. The distribution and duration of SARS-CoV-2 mRNA vaccine persistence in human tissues is unclear. Here, we developed specific RT-qPCR-based assays to detect each mRNA vaccine and screened lymph nodes, liver, spleen, and myocardium from recently vaccinated deceased patients. Vaccine was detected in the axillary lymph nodes in the majority of patients dying within 30 days of vaccination, but not in patients dying more than 30 days from vaccination. Vaccine was not detected in the mediastinal lymph nodes, spleen, or liver. Vaccine was detected in the myocardium in a subset of patients vaccinated within 30 days of death. Cardiac ventricles in which vaccine was detected had healing myocardial injury at the time of vaccination and had more myocardial macrophages than the cardiac ventricles in which vaccine was not detected. These results suggest that SARS-CoV-2 mRNA vaccines routinely persist up to 30 days from vaccination and can be detected in the heart.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

