Point-of-care testing with Xpert HPV for single-visit, screen-and-treat for cervical cancer prevention: a demonstration study in South Africa
- PMID: 37758811
- PMCID: PMC10533854
- DOI: 10.1038/s41598-023-43467-2
Point-of-care testing with Xpert HPV for single-visit, screen-and-treat for cervical cancer prevention: a demonstration study in South Africa
Abstract
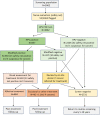
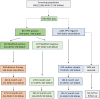
Human papillomavirus (HPV)-based screen-and-treat (SAT) is recommended but implementation presents operational challenges. We implemented HPV-SAT at a research site in Khayelitsha, South Africa, screening 3062 women aged 30-65 years (44% women living with HIV [WHIV]). All were screened using point-of-care Xpert HPV and almost all received their HPV results on the same day. HPV-positivity occurred in 41.5% of WHIV and 17.4% of women without HIV (WNoH) reducing to 26.2% in WHIV and 10.4% in WNoH applying treatment eligibility criteria based on high viral load in the channels detecting HPV16, 18, 45, 16, 18, 31, 33, 35, 52, 58. Among those eligible for treatment, 91.3% were considered suitable for ablative therapy, and 94.6% underwent thermal ablation on the same day, with no serious adverse events. Twelve months later, 39.0% of WHIV and 65.2% of WNoH treated with ablative therapy were clear of HPV. In women who were HPV-positive but ineligible for treatment, 19.1% and 12.9% had histologically-confirmed cervical intraepithelial neoplasia grade 2 or worse (CIN2+) at 12 months. SAT programs need to weigh trade-offs between overtreatment versus delayed or no treatment for women who test positive for HPV. Treatment modalities for precancerous lesions need to be improved.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Ferlay, J. et al. Global Cancer Observatory: Cancer today. https://gco.iarc.fr/today/home (Accessed 26 Sept 2023).
-
- World Health Organization. Global strategy to accelerate the elimination of cervical cancer as a public health problem. (2020).

