Objective measures of smoking and caffeine intake and the risk of adverse pregnancy outcomes
- PMID: 37759082
- PMCID: PMC10749751
- DOI: 10.1093/ije/dyad123
Objective measures of smoking and caffeine intake and the risk of adverse pregnancy outcomes
Abstract
Background: In pregnancy, women are encouraged to cease smoking and limit caffeine intake. We employed objective definitions of smoking and caffeine exposure to assess their association with adverse outcomes.
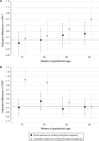
Methods: We conducted a case cohort study within the Pregnancy Outcome Prediction study to analyse maternal serum metabolomics in samples from 12, 20, 28 and 36 weeks of gestational age. Objective smoking status was defined based on detectable cotinine levels at each time point and objective caffeine exposure was based on tertiles of paraxanthine levels at each time point. We used logistic and linear regression to examine the association between cotinine, paraxanthine and the risk of pre-eclampsia, spontaneous pre-term birth (sPTB), fetal growth restriction (FGR), gestational diabetes mellitus and birthweight.
Results: There were 914 and 915 women in the smoking and caffeine analyses, respectively. Compared with no exposure to smoking, consistent exposure to smoking was associated with an increased risk of sPTB [adjusted odds ratio (aOR) = 2.58, 95% CI: 1.14 to 5.85)] and FGR (aOR = 4.07, 95% CI: 2.14 to 7.74) and lower birthweight (β = -387 g, 95% CI: -622 g to -153 g). On univariate analysis, consistently high levels of paraxanthine were associated with an increased risk of FGR but that association attenuated when adjusting for maternal characteristics and objective-but not self-reported-smoking status.
Conclusions: Based on objective data, consistent exposure to smoking throughout pregnancy was strongly associated with sPTB and FGR. High levels of paraxanthine were not independently associated with any of the studied outcomes and were confounded by smoking.
Keywords: Smoking; birthweight; caffeine; cotinine; fetal growth restriction; gestational diabetes; paraxanthine; pre-eclampsia; pre-term birth.
© The Author(s) 2023. Published by Oxford University Press on behalf of the International Epidemiological Association.
Conflict of interest statement
G.C.S.S. and D.S.C.-J. have received research support from Roche Diagnostics Ltd, Illumina and Sera Prognostics (FGR, PE and pre-term birth). G.C.S.S.’s department has received payment from Roche for a talk given by G.S. (FGR). G.C.S.S. has been a paid consultant to GSK (pre-term birth) and is a member of a Data Monitoring Committee for GSK trials of RSV vaccination in pregnancy. Current or recent government or charity grant support: MRC, BBSRC, NIHR, Wellcome Trust & Wellcome Leap.
Figures
References
-
- Yang L, Feng L, Huang L. et al. Maternal factors for intrauterine growth retardation: systematic review and meta-analysis of observational studies. Reprod Sci 2023;30:1737–45. - PubMed
-
- Di H-K, Gan Y, Lu K. et al. Maternal smoking status during pregnancy and low birth weight in offspring: systematic review and meta-analysis of 55 cohort studies published from 1986 to 2020. World J Pediatr 2022;18:176–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

