Effects of In Utero PFOS Exposure on Epigenetics and Metabolism in Mouse Fetal Livers
- PMID: 37759171
- PMCID: PMC10569047
- DOI: 10.1021/acs.est.3c05207
Effects of In Utero PFOS Exposure on Epigenetics and Metabolism in Mouse Fetal Livers
Abstract
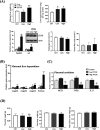
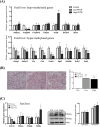
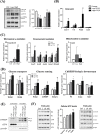
Prenatal exposure to perfluorooctanesulfonate (PFOS) increases fetus' metabolic risk; however, the investigation of the underlying mechanism is limited. In this study, pregnant mice in the gestational days (GD, 4.5-17.5) were exposed to PFOS (0.3 and 3 μg/g of body weight). At GD 17.5, PFOS perturbed maternal lipid metabolism and upregulated metabolism-regulating hepatokines (Angptl4, Angptl8, and Selenop). Mass-spectrometry imaging and whole-genome bisulfite sequencing revealed, respectively, selective PFOS localization and deregulation of gene methylation in fetal livers, involved in inflammation, glucose, and fatty acid metabolism. PCR and Western blot analysis of lipid-laden fetal livers showed activation of AMPK signaling, accompanied by significant increases in the expression of glucose transporters (Glut2/4), hexose-phosphate sensors (Retsat and ChREBP), and the key glycolytic enzyme, pyruvate kinase (Pk) for glucose catabolism. Additionally, PFOS modulated the expression levels of PPARα and PPARγ downstream target genes, which simultaneously stimulated fatty acid oxidation (Cyp4a14, Acot, and Acox) and lipogenesis (Srebp1c, Acaca, and Fasn). Using human normal hepatocyte (MIHA) cells, the underlying mechanism of PFOS-elicited nuclear translocation of ChREBP, associated with a fatty acid synthesizing pathway, was revealed. Our finding implies that in utero PFOS exposure altered the epigenetic landscape associated with dysregulation of fetal liver metabolism, predisposing postnatal susceptibility to metabolic challenges.
Keywords: AMPK; ChREBP; MIHA; MS-imaging; PPAR; hepatokine; whole-genome bisulfite sequencing.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Frisbee S. J.; Brooks A. P. Jr.; Maher A.; Flensborg P.; Arnold S.; Fletcher T.; Steenland K.; Shankar A.; Knox S. S.; Pollard C.; Halverson J. A.; Vieira V. M.; Jin C.; Leyden K. M.; Ducatman A. M. The C8 health project: design, methods, and participants. Environ. Health Perspect. 2009, 117 (12), 1873–1882. 10.1289/ehp.0800379. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

