Glycolytic shift during West Nile virus infection provides new therapeutic opportunities
- PMID: 37759218
- PMCID: PMC10537838
- DOI: 10.1186/s12974-023-02899-3
Glycolytic shift during West Nile virus infection provides new therapeutic opportunities
Abstract
Background: Viral rewiring of host bioenergetics and immunometabolism may provide novel targets for therapeutic interventions against viral infections. Here, we have explored the effect on bioenergetics during the infection with the mosquito-borne flavivirus West Nile virus (WNV), a medically relevant neurotropic pathogen causing outbreaks of meningitis and encephalitis worldwide.
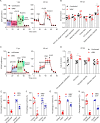
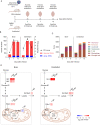
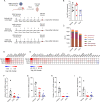
Results: A systematic literature search and meta-analysis pointed to a misbalance of glucose homeostasis in the central nervous system of WNV patients. Real-time bioenergetic analyses confirmed upregulation of aerobic glycolysis and a reduction of mitochondrial oxidative phosphorylation during viral replication in cultured cells. Transcriptomics analyses in neural tissues from experimentally infected mice unveiled a glycolytic shift including the upregulation of hexokinases 2 and 3 (Hk2 and Hk3) and pyruvate dehydrogenase kinase 4 (Pdk4). Treatment of infected mice with the Hk inhibitor, 2-deoxy-D-glucose, or the Pdk4 inhibitor, dichloroacetate, alleviated WNV-induced neuroinflammation.
Conclusions: These results highlight the importance of host energetic metabolism and specifically glycolysis in WNV infection in vivo. This study provides proof of concept for the druggability of the glycolytic pathway for the future development of therapies to combat WNV pathology.
Keywords: Glycolysis; Immunometabolism; Neuroinflammation; West Nile virus.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors report there are no competing interests to declare.
Figures

Similar articles
-
West Nile Virus-Inclusive Single-Cell RNA Sequencing Reveals Heterogeneity in the Type I Interferon Response within Single Cells.J Virol. 2019 Mar 5;93(6):e01778-18. doi: 10.1128/JVI.01778-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626670 Free PMC article.
-
Intrinsic Innate Immune Responses Control Viral Growth and Protect against Neuronal Death in an Ex Vivo Model of West Nile Virus-Induced Central Nervous System Disease.J Virol. 2021 Aug 25;95(18):e0083521. doi: 10.1128/JVI.00835-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34190599 Free PMC article.
-
Dynamics of Tissue-Specific CD8+ T Cell Responses during West Nile Virus Infection.J Virol. 2018 Apr 27;92(10):e00014-18. doi: 10.1128/JVI.00014-18. Print 2018 May 15. J Virol. 2018. PMID: 29514902 Free PMC article.
-
West Nile Disease Epidemiology in North-West Africa: Bibliographical Review.Transbound Emerg Dis. 2016 Dec;63(6):e153-e159. doi: 10.1111/tbed.12341. Epub 2015 Mar 6. Transbound Emerg Dis. 2016. PMID: 25753775 Review.
-
Interleukins, Chemokines, and Tumor Necrosis Factor Superfamily Ligands in the Pathogenesis of West Nile Virus Infection.Viruses. 2023 Mar 22;15(3):806. doi: 10.3390/v15030806. Viruses. 2023. PMID: 36992514 Free PMC article. Review.
Cited by
-
Mitochondrial Oxidative Phosphorylation in Viral Infections.Viruses. 2023 Dec 4;15(12):2380. doi: 10.3390/v15122380. Viruses. 2023. PMID: 38140621 Free PMC article. Review.
-
Reprogramming of liver metabolism during West Nile virus infection unveils novel aspects of disease pathophysiology.Mol Med. 2025 Jul 5;31(1):251. doi: 10.1186/s10020-025-01300-8. Mol Med. 2025. PMID: 40618034 Free PMC article.
-
The Antiviral Potential of AdipoRon, an Adiponectin Receptor Agonist, Reveals the Ability of Zika Virus to Deregulate Adiponectin Receptor Expression.Viruses. 2023 Dec 22;16(1):24. doi: 10.3390/v16010024. Viruses. 2023. PMID: 38257725 Free PMC article.
-
Changes in metabolite profiles in the cerebrospinal fluid and in human neuronal cells upon tick-borne encephalitis virus infection.J Neuroinflammation. 2025 Jun 14;22(1):157. doi: 10.1186/s12974-025-03478-4. J Neuroinflammation. 2025. PMID: 40517242 Free PMC article.
-
Alteration of mitochondrial function in arthropods during arboviruses infection: a review of the literature.Front Physiol. 2025 Feb 13;16:1507059. doi: 10.3389/fphys.2025.1507059. eCollection 2025. Front Physiol. 2025. PMID: 40017802 Free PMC article. Review.
References
-
- Barzon L. Ongoing and emerging arbovirus threats in Europe. J Clin Virol. 2018;107:38–47. - PubMed
-
- Musso D, Rodriguez-Morales AJ, Levi JE, Cao-Lormeau VM, Gubler DJ. Unexpected outbreaks of arbovirus infections: lessons learned from the Pacific and tropical America. Lancet Infect Dis. 2018;18:e355–e361. - PubMed
Publication types
MeSH terms
Grants and funding
- PRE2020-093374/Agencia Estatal de Investigación
- PID2019-105117RR-C21/Agencia Estatal de Investigación
- PID2019-105117RR-C22/Agencia Estatal de Investigación
- PID2019-105117RR-C21/Agencia Estatal de Investigación
- PID2020-119195RJ-I00/Agencia Estatal de Investigación
- PID2019-105117RR-C21/Agencia Estatal de Investigación
- PID2019-105117RR-C22/Agencia Estatal de Investigación
- PID2019-105117RR-C21/Agencia Estatal de Investigación
- NextGenerationEU through CSIC's Global Health Platform (PTI Salud Global)/European Commission
- NextGenerationEU through CSIC's Global Health Platform (PTI Salud Global)/European Commission
- NextGenerationEU through CSIC's Global Health Platform (PTI Salud Global)/European Commission
- NextGenerationEU through CSIC's Global Health Platform (PTI Salud Global)/European Commission
- NextGenerationEU through CSIC's Global Health Platform (PTI Salud Global)/European Commission
- NextGenerationEU through CSIC's Global Health Platform (PTI Salud Global)/European Commission
- NextGenerationEU through CSIC's Global Health Platform (PTI Salud Global)/European Commission
- NUTRISION-CM/Y2020/BIO-6350/Comunidad de Madrid
- NUTRISION-CM/Y2020/BIO-6350/Comunidad de Madrid
- NUTRISION-CM/Y2020/BIO-6350/Comunidad de Madrid
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

