Malaria vaccine coverage estimation using age-eligible populations and service user denominators in Kenya
- PMID: 37759277
- PMCID: PMC10523632
- DOI: 10.1186/s12936-023-04721-0
Malaria vaccine coverage estimation using age-eligible populations and service user denominators in Kenya
Abstract
Background: The World Health Organization approved the RTS,S/AS01 malaria vaccine for wider rollout, and Kenya participated in a phased pilot implementation from 2019 to understand its impact under routine conditions. Vaccine delivery requires coverage measures at national and sub-national levels to evaluate progress over time. This study aimed to estimate the coverage of the RTS,S/AS01 vaccine during the first 36 months of the Kenyan pilot implementation.
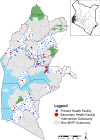
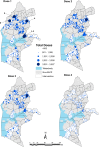
Methods: Monthly dose-specific immunization data for 23 sub-counties were obtained from routine health information systems at the facility level for 2019-2022. Coverage of each RTS,S/AS01 dose was determined using reported doses as a numerator and service-based (Penta 1 and Measles) or population (projected infant populations from WorldPop) as denominators. Descriptive statistics of vaccine delivery, dropout rates and coverage estimates were computed across the 36-month implementation period.
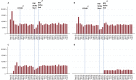
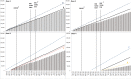
Results: Over 36 months, 818,648 RTSS/AS01 doses were administered. Facilities managed by the Ministry of Health and faith-based organizations accounted for over 88% of all vaccines delivered. Overall, service-based malaria vaccine coverage was 96%, 87%, 78%, and 39% for doses 1-4 respectively. Using a population-derived denominator for age-eligible children, vaccine coverage was 78%, 68%, 57%, and 24% for doses 1-4, respectively. Of the children that received measles dose 1 vaccines delivered at 9 months (coverage: 95%), 82% received RTSS/AS01 dose 3, only 66% of children who received measles dose 2 at 18 months (coverage: 59%) also received dose 4.
Conclusion: The implementation programme successfully maintained high levels of coverage for the first three doses of RTSS/AS01 among children defined as EPI service users up to 9 months of age but had much lower coverage within the community with up to 1 in 5 children not receiving the vaccine. Consistent with vaccines delivered over the age of 1 year, coverage of the fourth malaria dose was low. Vaccine uptake, service access and dropout rates for malaria vaccines require constant monitoring and intervention to ensure maximum protection is conferred.
Keywords: Kenya; Malaria; Malaria vaccine pilot; RTS,S/AS01; Vaccine coverage.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
EAO serves on the AstraZeneca Vaccine & Immune Therapies Effectiveness Evidence Scientific Advisory Committee (VEE-SAC).
Figures
References
-
- WHO . World malaria report 2022. Geneva: World Health Organization; 2022.
-
- WHO . Global technical strategy for malaria 2016-2030, 2021 update. Geneva: World Health Organization; 2021. pp. 1–40.
-
- Hawkes N. European medicines agency approves first malaria vaccine. BMJ. 2015;351:h4067. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

