Rectal progesterone administration secures a high ongoing pregnancy rate in a personalized Hormone Replacement Therapy Frozen Embryo Transfer (HRT-FET) protocol: a prospective interventional study
- PMID: 37759346
- PMCID: PMC10628493
- DOI: 10.1093/humrep/dead185
Rectal progesterone administration secures a high ongoing pregnancy rate in a personalized Hormone Replacement Therapy Frozen Embryo Transfer (HRT-FET) protocol: a prospective interventional study
Abstract
Study question: Can supplementation with rectal administration of progesterone secure high ongoing pregnancy rates (OPRs) in patients with low serum progesterone (P4) on the day of blastocyst transfer (ET)?
Summary answer: Rectally administered progesterone commencing on the ET day secures high OPRs in patients with serum P4 levels below 35 nmol/l (11 ng/ml).
What is known already: Low serum P4 levels at peri-implantation in Hormone Replacement Therapy Frozen Embryo Transfer (HRT-FET) cycles impact reproductive outcomes negatively. However, studies have shown that patients with low P4 after a standard vaginal progesterone treatment can obtain live birth rates (LBRs) comparable to patients with optimal P4 levels if they receive additionalsubcutaneous progesterone, starting around the day of blastocyst transfer. In contrast, increasing vaginal progesterone supplementation in low serum P4 patients does not increase LBR. Another route of administration rarely used in ART is the rectal route, despite the fact that progesterone is well absorbed and serum P4 levels reach a maximum level after ∼2 h.
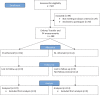
Study design, size, duration: This prospective interventional study included a cohort of 488 HRT-FET cycles, in which a total of 374 patients had serum P4 levels ≥35 nmol/l (11 ng/ml) at ET, and 114 patients had serum P4 levels <35 nmol/l (11 ng/ml). The study was conducted from January 2020 to November 2022.
Participants/materials, setting, methods: Patients underwent HRT-FET in a public Fertility Clinic, and endometrial preparation included oral oestradiol (6 mg/24 h), followed by vaginal micronized progesterone, 400 mg/12 h. Blastocyst transfer and P4 measurements were performed on the sixth day of progesterone administration. In patients with serum P4 <35 nmol/l (11 ng/ml), 'rescue' was performed by rectal administration of progesterone (400 mg/12 h) starting that same day. In pregnant patients, rectal administration continued until Week 8 of gestation, and oestradiol and vaginal progesterone treatment continued until Week 10 of gestation.
Main results and the role of chance: Among 488 HRT-FET single blastocyst transfers, the mean age of the patients at oocyte retrieval (OR) was 30.9 ± 4.6 years and the mean BMI at ET 25.1 ± 3.5 kg/m2. The mean serum P4 level after vaginal progesterone administration on the day of ET was 48.9 ± 21.0 nmol/l (15.4 ± 6.6 ng/ml), and a total of 23% (114/488) of the patients had a serum P4 level lower than 35 nmol/l (11 ng/ml). The overall, positive hCG rate, clinical pregnancy rate, OPR week 12, and total pregnancy loss rate were 66% (320/488), 54% (265/488), 45% (221/488), and 31% (99/320), respectively. There was no significant difference in either OPR week 12 or total pregnancy loss rate between patients with P4 ≥35 nmol/l (11 ng/ml) and patients with P4 <35 nmol/l, who received rescue in terms of rectally administered progesterone, 45% versus 46%, P = 0.77 and 30% versus 34%, P = 0.53, respectively. OPR did not differ whether patients had initially low P4 and rectal rescue or were above the P4 cut-off. Logistic regression analysis showed that only age at OR and blastocyst scoring correlated with OPR week 12, independently of other factors like BMI and vitrification day of blastocysts (Day 5 or 6).
Limitations, reasons for caution: In this study, vaginal micronized progesterone pessaries, a solid pessary with progesterone suspended in vegetable hard fat, were used vaginally as well as rectally. It is unknown whether other vaginal progesterone products, such as capsules, gel, or tablet, could be used rectally with the same rescue effect.
Wider implications of the findings: A substantial part of HRT-FET patients receiving vaginal progesterone treatment has lowserum P4. Adding rectally administered progesterone in these patients increases the reproductive outcome. Importantly, rectal progesterone administration is considered convenient, and progesterone pessaries are easy to administer rectally and of low cost.
Study funding/competing interest(s): Gedeon Richter Nordic supported the study with an unrestricted grant as well as study medication. B.A. has received unrestricted grant from Gedeon Richter Nordic and Merck and honoraria for lectures from Gedeon Richter, Merck, IBSA and Marckyrl Pharma. P.H. has received honoraria for lectures from Gedeon Richter, Merck, IBSA and U.S.K. has received grant from Gedeon Richter Nordic, IBSA and Merck for studies outside this work and honoraria for teaching from Merck and Thillotts Pharma AB and conference expenses covered by Merck. The other co-authors have no conflict of interest to declare.
Trial registration number (25): EudraCT no.: 2019-001539-29.
Keywords: HRT-FET; low serum progesterone; luteal phase rescue; ongoing pregnancy; rectal progesterone; vaginal progesterone.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
B.A. has received unrestricted grant from Gedeon Richter Nordic and Merck and honoraria for lectures from Gedeon Richter, Merck, IBSA and Marckyrl Pharma. P.H. has received honoraria for lectures from Gedeon Richter, Merck, IBSA and U.S.K. has received grant from Gedeon Richter Nordic, IBSA and Merck for studies outside this work and honoraria for teaching from Merck and Thillotts Pharma AB and conference expenses covered by Merck. The other co-authors have no conflict of interest to declare.
Figures
Similar articles
-
Early pregnancy bleeding after assisted reproductive technology: a systematic review and secondary data analysis from 320 patients undergoing hormone replacement therapy frozen embryo transfer.Hum Reprod. 2023 Dec 4;38(12):2373-2381. doi: 10.1093/humrep/dead218. Hum Reprod. 2023. PMID: 37897214
-
The impact of luteal serum progesterone levels on live birth rates-a prospective study of 602 IVF/ICSI cycles.Hum Reprod. 2018 Aug 1;33(8):1506-1516. doi: 10.1093/humrep/dey226. Hum Reprod. 2018. PMID: 29955789
-
A drop in serum progesterone from oocyte pick-up +3 days to +5 days in fresh blastocyst transfer, using hCG-trigger and standard luteal support, is associated with lower ongoing pregnancy rates.Hum Reprod. 2023 Feb 1;38(2):225-236. doi: 10.1093/humrep/deac255. Hum Reprod. 2023. PMID: 36478179
-
Does luteal phase progesterone supplementation affect physical and psychosocial well-being among women undergoing modified natural cycle-FET? A sub-study of a randomized controlled trial.Hum Reprod. 2023 Oct 3;38(10):1970-1980. doi: 10.1093/humrep/dead171. Hum Reprod. 2023. PMID: 37634089 Clinical Trial.
-
Day 5 versus Day 6 blastocyst transfers: a systematic review and meta-analysis of clinical outcomes.Hum Reprod. 2019 Oct 2;34(10):1948-1964. doi: 10.1093/humrep/dez163. Hum Reprod. 2019. PMID: 31644803 Free PMC article.
Cited by
-
Corpus luteum and progesterones in embryo transfer cycles: current challenges of different luteal phase support protocols.JBRA Assist Reprod. 2024 Jun 1;28(2):211-214. doi: 10.5935/1518-0557.20240044. JBRA Assist Reprod. 2024. PMID: 38775322 Free PMC article. No abstract available.
-
Rectal versus vaginal progesterone administration for luteal phase support in the hormone replacement therapy frozen embryo transfer (HRT-FET) cycle: protocol for a non-inferiority randomised controlled trial.BMJ Open. 2024 Jul 3;14(7):e082879. doi: 10.1136/bmjopen-2023-082879. BMJ Open. 2024. PMID: 38960462 Free PMC article.
-
Intensive luteal phase support in hormone replacement and modified natural cycle frozen embryo transfers in ovulatory patients: A propensity score-matched study.PLoS One. 2025 Jul 17;20(7):e0327470. doi: 10.1371/journal.pone.0327470. eCollection 2025. PLoS One. 2025. PMID: 40674314 Free PMC article.
References
-
- Aghsa MM, Rahmanpour H, Bagheri M, Davari-Tanha F, Nasr R.. A randomized comparison of the efficacy, side effects and patient convenience between vaginal and rectal administration of Cyclogest((R)) when used for luteal phase support in ICSI treatment. Arch Gynecol Obstet 2012;286:1049–1054. - PubMed
-
- Alsbjerg B, Kesmodel US, Elbaek HO, Laursen R, Laursen SB, Andreasen D, Povlsen BB, Humaidan P.. GnRH agonist supplementation in hormone replacement therapy-frozen embryo transfer cycles: a randomized controlled trial. Reprod Biomed Online 2021;44:261–270. - PubMed
-
- Alsbjerg B, Kesmodel US, Humaidan P.. Endometriosis patients benefit from high serum progesterone in hormone replacement therapy-frozen embryo transfer cycles: a cohort study. Reprod Biomed Online 2023;46:92–98. - PubMed
-
- Alsbjerg B, Thomsen L, Elbaek HO, Laursen R, Povlsen BB, Haahr T, Humaidan P.. Progesterone levels on pregnancy test day after hormone replacement therapy-cryopreserved embryo transfer cycles and related reproductive outcomes. Reprod Biomed Online 2018;37:641–647. - PubMed
-
- Alsbjerg B, Thomsen L, Elbaek HO, Laursen R, Povlsen BB, Haahr T, Humaidan P.. Can combining vaginal and rectal progesterone achieve the optimum progesterone range required for implantation in the HRT-FET model? Reprod Biomed Online 2020;40:805–811. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials