Diverse Roles of Protein Palmitoylation in Cancer Progression, Immunity, Stemness, and Beyond
- PMID: 37759431
- PMCID: PMC10526800
- DOI: 10.3390/cells12182209
Diverse Roles of Protein Palmitoylation in Cancer Progression, Immunity, Stemness, and Beyond
Abstract
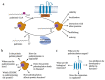
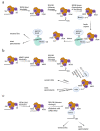
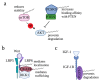
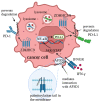
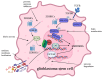
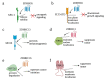
Protein S-palmitoylation, a type of post-translational modification, refers to the reversible process of attachment of a fatty acyl chain-a 16-carbon palmitate acid-to the specific cysteine residues on target proteins. By adding the lipid chain to proteins, it increases the hydrophobicity of proteins and modulates protein stability, interaction with effector proteins, subcellular localization, and membrane trafficking. Palmitoylation is catalyzed by a group of zinc finger DHHC-containing proteins (ZDHHCs), whereas depalmitoylation is catalyzed by a family of acyl-protein thioesterases. Increasing numbers of oncoproteins and tumor suppressors have been identified to be palmitoylated, and palmitoylation is essential for their functions. Understanding how palmitoylation influences the function of individual proteins, the physiological roles of palmitoylation, and how dysregulated palmitoylation leads to pathological consequences are important drivers of current research in this research field. Further, due to the critical roles in modifying functions of oncoproteins and tumor suppressors, targeting palmitoylation has been used as a candidate therapeutic strategy for cancer treatment. Here, based on recent literatures, we discuss the progress of investigating roles of palmitoylation in regulating cancer progression, immune responses against cancer, and cancer stem cell properties.
Keywords: ZDHHCs; cancer; cancer treatment; palmitoylation; protein post-translational modification; tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

